Tenascin C activates the toll‑like receptor 4/NF‑κB signaling pathway to promote the development of polycystic ovary syndrome
- Authors:
- Published online on: April 25, 2024 https://doi.org/10.3892/mmr.2024.13230
- Article Number: 106
-
Copyright: © Wu et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Polycystic ovary syndrome (PCOS), a common gynecological disease associated with both endocrine and metabolic disorders, has become a leading cause of anovulatory infertility among women of reproductive age globally (1,2). The pathophysiology associated with PCOS is multifaceted, including numerous characteristics such as hypothalamic-pituitary-ovarian axis dysfunction, which is induced by defects in steroidogenesis, insulin resistance (IR), fat deposition and hyperandrogenism (3). Approximately 75% of PCOS cases are associated with IR (4). Metabolic abnormalities, chronic inflammation and oxidative stress have been demonstrated to be related to IR in PCOS (5,6). Although PCOS research began in 1953, its heterogeneity has hindered a complete understanding of its pathogenesis (7). Therefore, elucidating PCOS pathogenesis will help improve the treatment of PCOS. Although there is no known cure for PCOS, there is an increasing interest in investigating the role of target genes in the diagnosis and management of PCOS (8–10).
Tenascin C (TNC) is an important extracellular matrix glycoprotein and has numerous effects on cellular responses, including cell adhesion, motility, differentiation and growth (11). TNC is expressed at low levels in normal adult tissues (11). Notably, TNC expression is upregulated in specific pathological conditions, including inflammation, infection, cancer and injury repair (12–14). Ishizaki et al (15) reported that TNC was a critical underlying indicator in the severity of antineutrophil cytoplasmic antibody-associated vasculitis. TNC has been demonstrated to accelerate hypoxia-induced cardiac injury in a methyltransferase 3, N6-adenosine-methyltransferase complex catalytic subunit-dependent manner (16). In ankylosing spondylitis, inflammation-induced aberrant expression of TNC accelerates entheseal new bone formation by repressing extracellular matrix adhesion forces and activating the Hippo signaling pathway (17). In addition, TNC and multiple molecular cascades have been reported to regulate cellular responses in inflammation, tissue repair and even myocardial regeneration (18). Dumesic et al (19) observed high TNC expression in patients with PCOS compared with control patients, indicating that TNC may serve a role in PCOS. Since the importance of inflammation has attracted attention in PCOS pathophysiology, the present study focused on the effects of TNC in PCOS.
The present study investigated the effect of TNC PCOS in vivo and in vitro. Then, it further studied the mechanism by which TNC promoted PCOS in vivo and in vitro. The present study may contribute to an improved understanding of the molecular mechanism of PCOS and provide a promising target for PCOS treatment.
Materials and methods
Bioinformatics analysis
The GSE124226 dataset in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) were analyzed for assessing TNC expression in PCOS patients and healthy people. To retrieve the signaling pathways regulated by TNC, the Gene Set Enrichment Analysis (GSEA) software (http://software.broadinstitute.org/gsea/index.jsp) was conducted using Kyoto Encyclopedia of Genes and Genomes (KEGG) datasets (c2.cp.kegg.v2023.1.Hs.symbols.gmt, http://www.gsea-msigdb.org/gsea/msigdb/human/genesets.jsp?collection=CP:KEGG_MEDICUS).
Rat model of PCOS
A total of 20 female Sprague-Dawley rats (3 weeks old, 50–60 g) were supplied by Jinan Pengyue Experimental Animal Breeding Co., Ltd. All rats were housed at a controlled temperature of 25±2°C in standard cages with 55±15% humidity under a 12-h light/dark cycle and given ad libitum access to sterilized food and water for 1 week before the experiments. All animal procedures were overseen and approved by the Ethical Committee of The Second Affiliated Hospital of Shandong First Medical University (no. 2021-128; Taian, China). Rats were randomly assigned to four groups (n=5 each): Control, PCOS, PCOS + short hairpin RNA (shRNA/sh)-negative control (NC) and PCOS + sh-TNC. A rat PCOS model was induced using dehydroepiandrosterone (DHEA). Briefly, rats in the PCOS group were subcutaneously injected daily with 60 mg/kg DHEA (MilliporeSigma) diluted with 0.2 ml sesame oil for 21 consecutive days. At the same time, the rats in the control group were injected with an equal volume of sesame oil (20–23). Prior to DHEA injection to induce PCOS in rats, lentivirus carrying sh-TNC or sh-NC (10 µl; Shanghai GeneChem Co., Ltd.) was injected into the ovaries of rats. The shRNA sequences were as follows: sh-TNC, 5′-CGACATGTTTACTATCGAA-3′ and scrambled sh-NC, 5′-TTCTCCGAACGTGTCACGT-3′. At 3 weeks after the lentivirus injection, blood and ovaries of the rats were collected for the subsequent analyses. The humane endpoints of the present study were as follows: i) At least 10% weight loss with <40% food and water intake within 7 days following DHEA treatment; and ii) at least 20% weight loss without food or water intake with repeated or prolonged convulsions or ataxia at the end of 21 days of DHEA treatment. The rats were sacrificed using intraperitoneal injection of 250 mg/kg pentobarbital sodium. After respiratory and cardiac arrest, monitoring was continued for a further 5 min to confirm animal death. The whole experiment, including a week of acclimation, lasted 29 days. No rats succumbed during the present study. The animal health and behavior were monitored daily.
Determination of hormone and pro-inflammatory cytokine levels
Rats were anesthetized by intraperitoneal injection of 50 mg/kg pentobarbital sodium (24–28), and then blood (2 ml) was collected from the abdominal aorta. After blood collection, the rats were euthanized immediately by intraperitoneal injection of 250 mg/kg pentobarbital sodium. After respiratory and cardiac arrest, monitoring was continued for a further 5 min to confirm animal death. Serum levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone, estradiol and insulin, as well as the levels of pro-inflammatory cytokines in ovarian tissues, were assessed using their corresponding ELISA kits (LH ELISA kit; cat. no. SEKR-0091; Beijing Solarbio Science & Technology Co., Ltd.; FSH ELISA kit, SEKR-0090; Beijing Solarbio Science & Technology Co., Ltd.; testosterone ELISA kit; cat. no. SEKSM-0003; Beijing Solarbio Science & Technology Co., Ltd.; estradiol ELISA kit; cat. no. SEKR-0107; Beijing Solarbio Science & Technology Co., Ltd.; insulin ELISA kit; cat. no. SEKR-0033; Beijing Solarbio Science & Technology Co., Ltd.; TNF-α ELISA kit; cat. no. EK0526; Wuhan Boster Biological Technology, Ltd.; IL-1β ELISA kit; cat. no. EK0393; Wuhan Boster Biological Technology, Ltd.; IL-6 ELISA kit; cat. no. EK0412; Wuhan Boster Biological Technology, Ltd.; IL-18 ELISA kit, EK0592; Wuhan Boster Biological Technology, Ltd.), according to the manufacturer's protocol.
IR assay
The IR was assessed by the homeostasis model assessment of IR (HOMA-IR) (29), which was calculated as follows: Fasting blood glucose (FBG) × fasting serum insulin (FINS)/22.5. The rats were fasted for 12 h after the last DHEA administration and the blood glucose level was measured utilizing a Glucose Assay Kit (Beyotime Institute of Biotechnology).
Histology and immunohistochemistry staining
After being harvested from rats, the ovarian tissues were fixed in 4% paraformaldehyde buffer for 24 h at room temperature, embedded in paraffin and cut into 4-µm-thick slices. The sections were stained using hematoxylin for 8 min and eosin for 5 min at room temperature. Morphological changes in ovarian tissues were observed under a light microscope.
For immunohistochemistry, the ovarian tissues were fixed in 4% paraformaldehyde buffer for 24 h at room temperature, embedded in paraffin and cut into 4-µm-thick slices. Following this, the slides were heated at 60°C, then dewaxed by xylene and rehydrated by fractionated ethanol. The sections were incubated in hydrogen peroxide (3%, vol/vol) in PBS for 20 min at 37°C to block the endogenous peroxidase activity. After blocking of nonspecific activity with 5% goat serum (Oumarsi Biotech) for 60 min at room temperature, the sections were incubated with primary antibodies, including TNC (1:50; cat. no. ab108930; Abcam), TLR4 (1:50; cat. no. 19811-1-AP; Proteintech Group, Inc.) and phosphorylated (p-)NF-κB (1:100; cat. no. SAB5700363; MilliporeSigma) antibodies, overnight at 4°C. Subsequently, the slices were incubated with a biotinylated secondary antibody (1:500; cat. no. ab7176; Abcam) for 1 h at room temperature. The sections were observed under a light microscope after staining with 3,3′-diaminobenzidine (MilliporeSigma) for 5 min at room temperature and counterstaining with hematoxylin for 60 sec at room temperature.
Measurement of malondialdehyde (MDA), reduced glutathione (GSH) and superoxide dismutase (SOD) levels
According to the manufacturer's protocol, the levels of MDA, GSH and SOD in ovarian tissues and cell culture supernatants were determined using MDA content assay kit (Beijing Solarbio Science & Technology Co., Ltd.), GSH content assay kit (Beijing Solarbio Science & Technology Co., Ltd.) and SOD activity assay kit (Beijing Solarbio Science & Technology Co., Ltd.), respectively.
Reverse transcription-quantitative PCR (RT-qPCR)
RNA was extracted from ovarian tissues and KGN cells using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was synthesized using the GoScript™ Reverse Transcription System (Promega Corporation) for 60 min at 42°C. RT and qPCR were performed using the Hifair III One-Step RT-qPCR SYBR Green Kit (Shanghai Yeasen Biotechnology Co., Ltd.). β-actin was used as the endogenous control. The thermocycling conditions were: 95°C for 5 min, followed by 40 cycles at 95°C for 10 sec and 60°C for 30 sec. The following primers were used: Rat TNC forward, 5′-TCTCTGGAATTGCTCCCAGC-3′ and reverse, 5′-TTCCGGTTCAGCTTCTGTGG-3′; human TNC forward, 5′-GGGGGTGGATGGATTGTGTT-3′ and reverse, 5′-CTTCTCTGCGGTCCCCAAAT-3′; human TNF-α forward, 5′-CCCCAGGGACCTCTCTCTAA-3′ and reverse, 5′-GCTTGAGGGTTTGCTACAACA-3′; human IL-1β forward, 5′-GCCCTAAACAGATGAAGTGCTC-3′ and reverse, 5′-CCATGGCCACAACAACTGAC-3′; human IL-6 forward, 5′-CTCCTTCTCCACAAGCGCC-3′ and reverse, 5′-GATGCCGTCGAGGATGTACC-3′; human IL-18 forward, 5′-TTGACCAAGGAAATCGGCCT-3′ and reverse, 5′-GTCCGGGGTGCATTATCTCT-3′; rat β-actin forward, 5′-GCCTTCCTTCCTGGGTATGG-3′ and reverse, 5′-AATGCCTGGGTACATGGTGG-3′; and human β-actin forward, 5′-GATTCCTATGTGGGCGACGA-3′ and reverse, 5′-AGGTCTCAAACATGATCTGGGT-3′. The 2−ΔΔCq method was used to calculate the mRNA expression level (30).
Western blotting
RIPA buffer (Thermo Fisher Scientific, Inc.) was applied to lyse the cells and ovarian tissues to obtain proteins. The protein was quantified using a BCA kit (Beyotime Institute of Biotechnology). Next, 10% SDS-PAGE was employed to separate the protein samples (50 µg). Subsequently, the protein bands were transferred onto a PVDF membrane (Bio-Rad Laboratories, Inc.). After blocking with 5% non-fat milk at room temperature for 1 h, the membranes were incubated with the corresponding primary antibodies, including TNC (1:500; cat. no. ab108930; Abcam), Bcl-2 (1:1,000; cat. no. ab196495; Abcam), cleaved caspase-3 (1:1,000; cat. no. 9661; Cell Signaling Technology, Inc.), TLR4 (1:1,000; cat. no. 19811-1-AP; Proteintech Group, Inc.), NF-κB (1:1,000; cat. no. 8242; Cell Signaling Technology, Inc.), p-NF-κB (1:500; cat. no. SAB5700363; MilliporeSigma) and anti-GAPDH (1:2,000; cat. no. ab181602; Abcam) antibodies, at 4°C overnight. After washing with TBST (TBS + 0.1% Tween-20) three times for 5 min each, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (1:5,000; cat. no. SA00001-2; Proteintech Group, Inc.) for 1 h at room temperature. Protein bands were visualized by incubation with an enhanced chemiluminescence reagent (Beyotime Institute of Biotechnology). Finally, the bands were quantified using Quantity One 4.1.1 gel analysis software (Bio-Rad Laboratories, Inc.). GAPDH was used as the loading control.
Cell treatments
KGN human granulosa cells were obtained from Procell Life Science & Technology Co., Ltd. and incubated in DMEM/F12 (Procell Life Science & Technology Co., Ltd.) at 37°C with 5% CO2. The medium contained 10% FBS (Thermo Fisher Scientific, Inc.) and 100 U/ml penicillin/streptomycin (Thermo Fisher Scientific, Inc.). The PCOS cell model was established by treating cells with dihydrotestosterone (DHT) solution (500 nM) for 24 h. Next, 100 nM small interfering RNA (siRNA/si)-TNC and 100 nM si-NC (both TsingKe Biological Technology) were transfected into KGN cells for 48 h at 37°C using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.). The medium was replaced with fresh medium and the cells were cultured for another 24 h. The siRNA sequences were as follows: si-TNC, sense 5′-CGGUGUAUAUUAAGUGCUAGU-3′ and antisense 5′-UAGCACUUAAUAUACACCGGG-3′; and si-NC, sense 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense 5′-ACGUGACACGUUCGGAGAATT-3′.
5-ethynyl-2′-deoxyuridine (EdU) assay
Proliferation was evaluated using an EdU Apollo DNA in vitro kit (Guangzhou RiboBio Co., Ltd.). Briefly, treated KGN cells (5×103 cells/well) were plated in 24-well plates and incubated for 24 h and cultured with 50 µM EdU for 120 min at room temperature. Following fixing with 4% paraformaldehyde for 30 min at room temperature and permeabilizing with 0.3% Triton X-100 for 10 min, KGN cells were stained with DAPI at room temperature in the dark for 20 min. The positive cells were observed under a fluorescence microscope and captured images were analyzed using ImageJ software v1.51 (National Institutes of Health).
Flow cytometry
The treated KGN cells were resuspended in binding buffer, and Annexin V-FITC (5 µl) and propidium iodide (5 µl) staining solutions (BD Biosciences) were added to the cell suspension. Next, the cells were incubated for 15 min at room temperature in a dark room, followed immediately by flow cytometry using a FACScan flow cytometry system (Becton, Dickinson and Company). The data were analysed using FlowJo software (v7.6.5; FlowJo, LLC).
Immunofluorescence assay
The treated KGN cells were washed using PBS, fixed in 4% paraformaldehyde for 30 min at room temperature, and permeabilized with 0.3% Triton X-100 on ice. After washing three times with PBS, the cells were blocked with 3% bovine serum albumin (MilliporeSigma) for half an hour at room temperature. Next, KGN cells were incubated at 4°C overnight with primary antibodies, including TLR4 (1:50; cat. no. 19811-1-AP; Proteintech Group, Inc.) and p-NF-κB (1:100; cat. no. SAB5700363; MilliporeSigma) antibodies. The following day, KGN cells were incubated with the goat anti-rabbit IgG H&L (Alexa Fluor® 488; cat. no. ab150077; 1:500; Abcam) for 2 h at room temperature, followed by washing with PBS. Finally, cells were counterstained with DAPI for 10 min at room temperature and images were captured under a fluorescence microscope.
Statistical analysis
SPSS 22.2 (IBM Corp.) and GraphPad Prism 8.0 (Dotmatics) were employed to perform statistical analyses. An unpaired Student's t-test or one-way or two-way ANOVA with Tukey's post hoc test was utilized for group comparisons. All experiments were repeated three times. Data are presented as the mean ± SD. P<0.05 was considered to indicate a statistically significant difference.
Results
TNC is highly expressed in PCOS rats
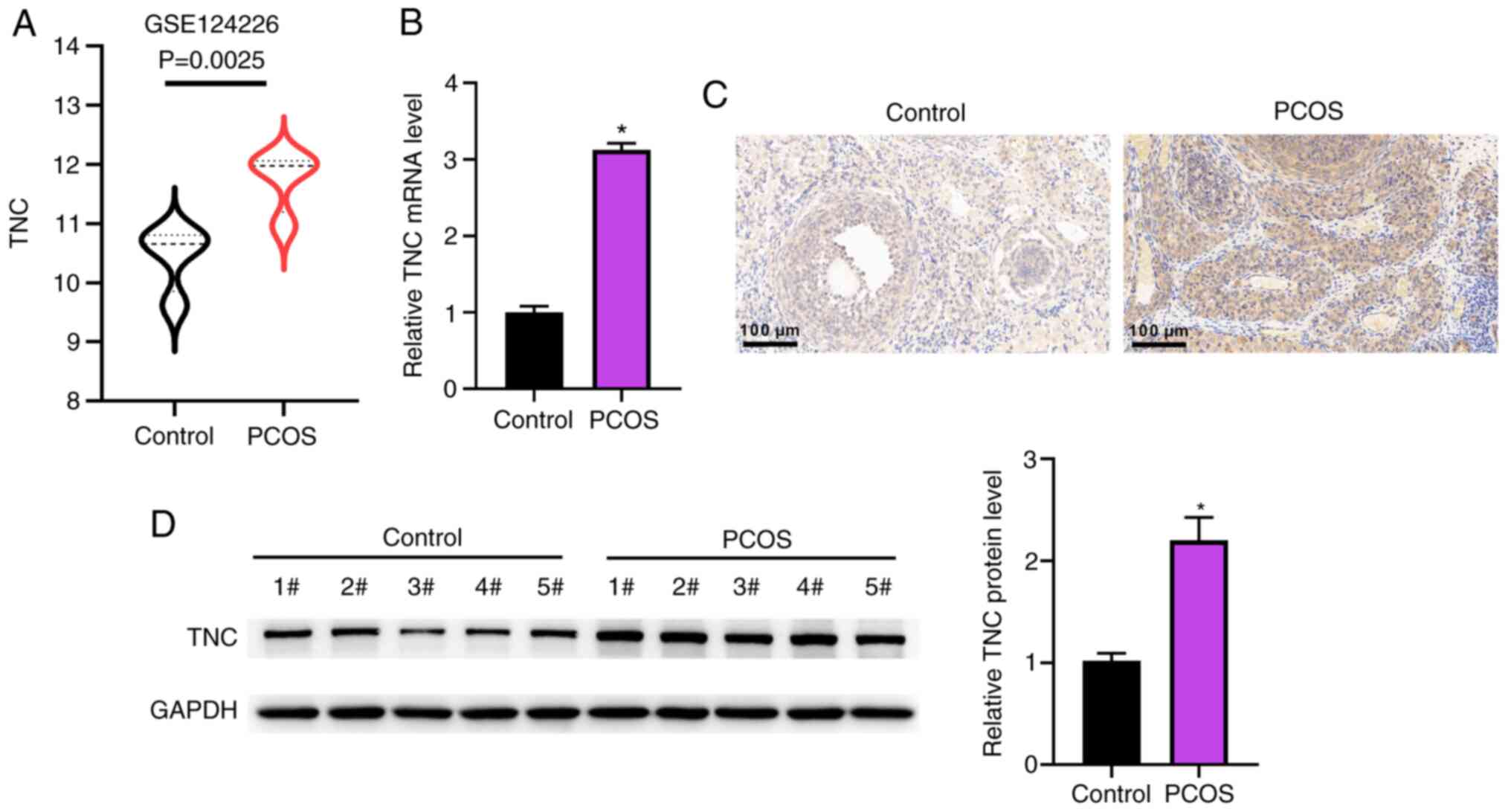
Based on analysis of the Gene Expression Omnibus dataset GSE124226, TNC mRNA expression was significantly upregulated in the patients with PCOS compared with the healthy controls (Fig. 1A). Additionally, the TNC mRNA levels were higher in PCOS rats than in control rats (Fig. 1B). Similarly, immunohistochemistry and western blotting further demonstrated that TNC protein expression was upregulated in the ovarian tissues of PCOS rats (Fig. 1C and D).
Knockdown of TNC relieves the pathological characteristics and the endocrine abnormalities of PCOS rats
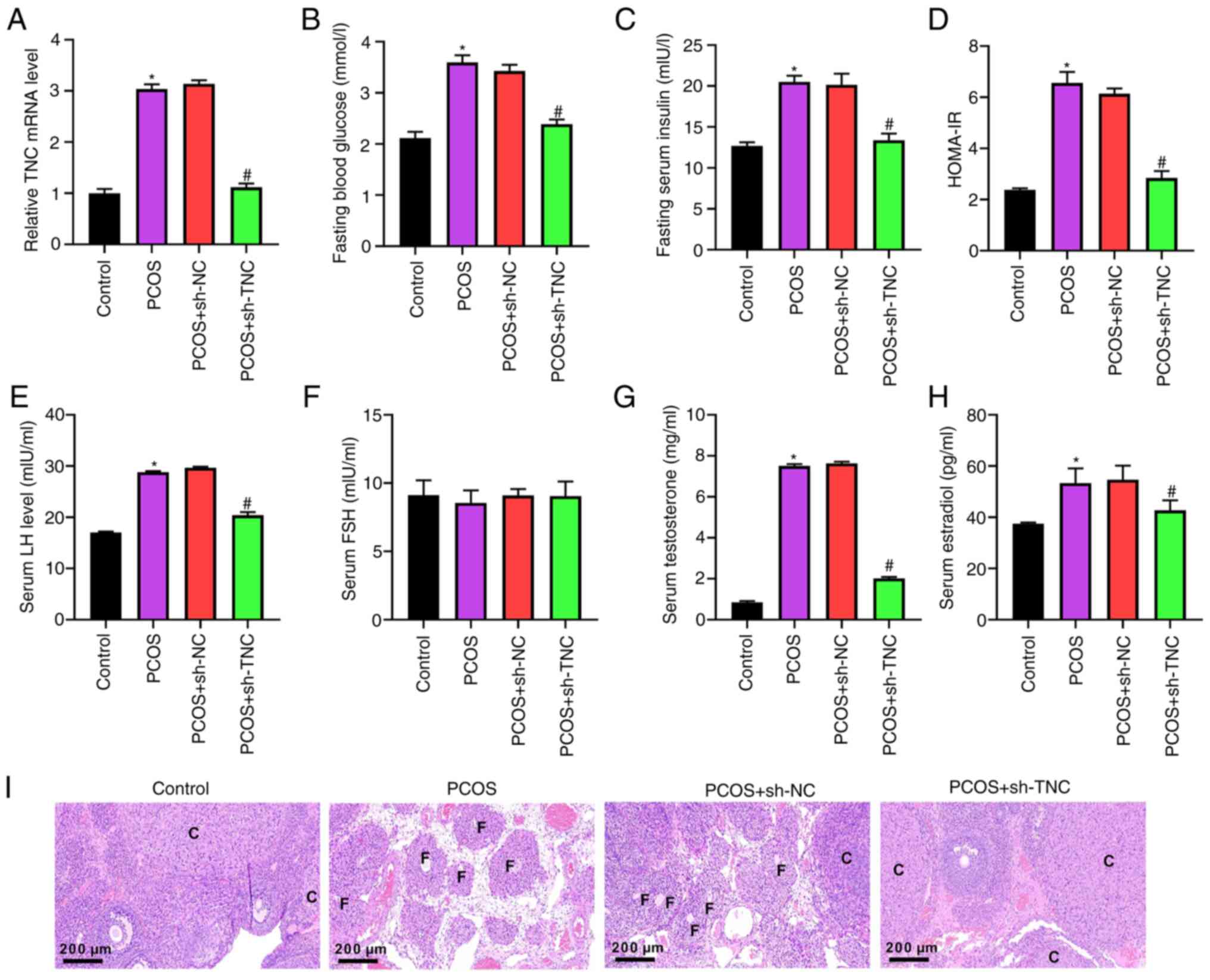
As shown in Fig. 2A, the TNC level was significantly increased in the PCOS group compared with the control group. Compared with that in the PCOS + sh-NC group, the TNC level was reduced in the PCOS + sh-TNC group (Fig. 2A). IR is a key indicator of PCOS (31). The concentrations of FBG and FINS, and the HOMA-IR index were elevated in the PCOS group compared with the control group (Fig. 2B-D). The elevated FBG and FINS concentrations, and HOMA-IR index were reduced by injection of sh-TNC (Fig. 2B-D). Additionally, knockdown of TNC markedly reversed the elevated LH, testosterone and estradiol levels induced by DHEA, but did not affect FSH (Fig. 2E-H). H&E staining was used to explore the morphological changes in the ovaries (Fig. 2I). Cystic dilatation of follicles, reduced corpus luteum, increased atretic follicles and a thin layer of granulosa cells were observed in the PCOS group compared with the control group (Fig. 2I). Notably, these phenotypes were improved in the group injected with sh-TNC (Fig. 2I).
Knockdown of TNC inhibits ovarian cell apoptosis, oxidative stress and inflammation in PCOS rats
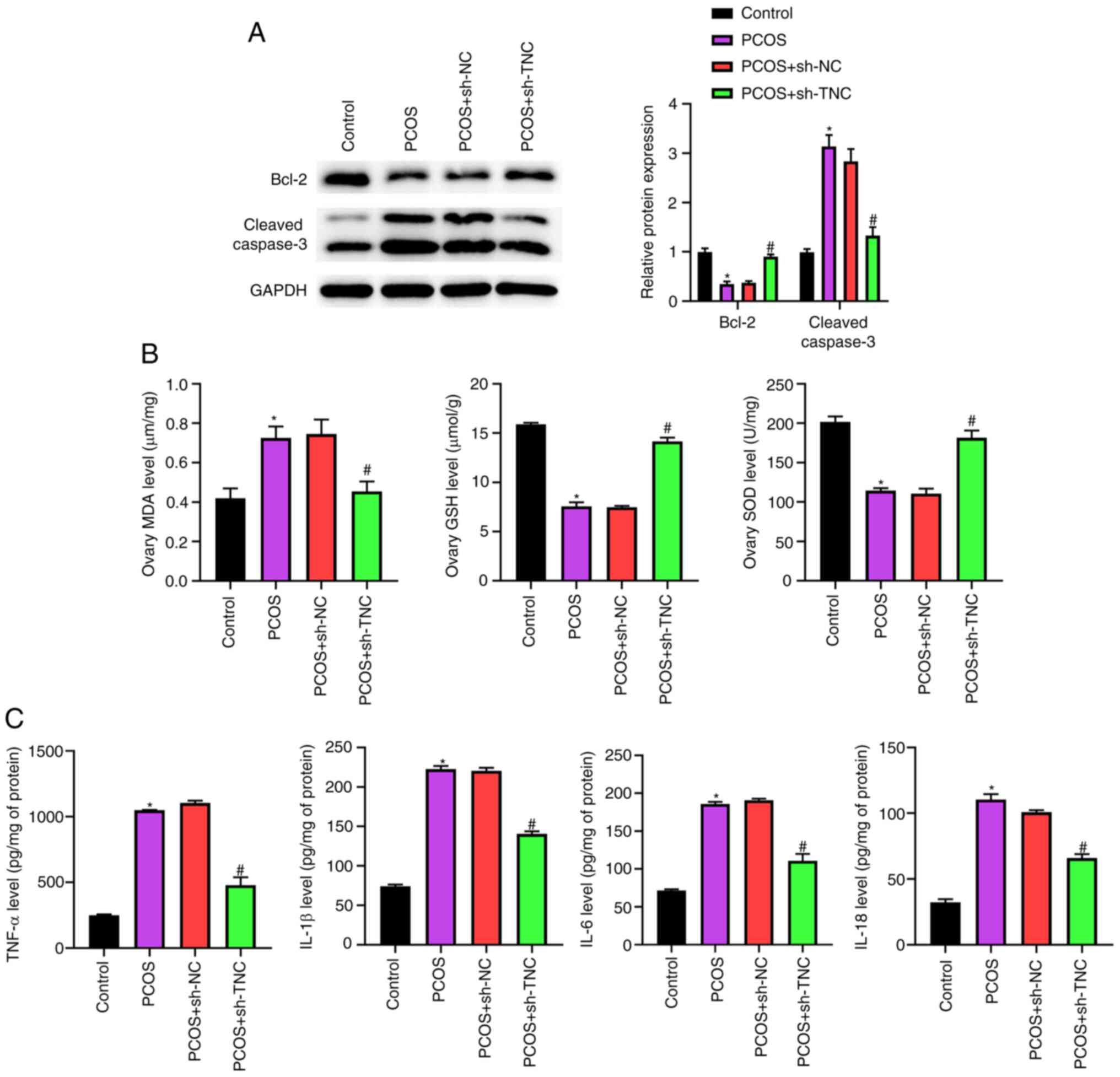
Western blotting demonstrated that knockdown of TNC significantly counteracted the decrease in Bcl-2 expression and the increase in cleaved caspase-3 expression caused by DHEA (Fig. 3A). Furthermore, upregulation of MDA and knockdown of GSH and SOD in PCOS rats were reversed by the injection of sh-TNC (Fig. 3B). The results of ELISAs showed that the elevated levels of TNF-α, IL-1β, IL-6 and IL-18 in PCOS rats were neutralized by the administration of sh-TNC (Fig. 3C). Overall, these data indicated that TNC knockdown could effectively inhibit ovarian cell apoptosis, oxidative stress and inflammation in PCOS rats.
Knockdown of TNC inhibits the activation of the TLR4/NF-κB pathway in PCOS rats
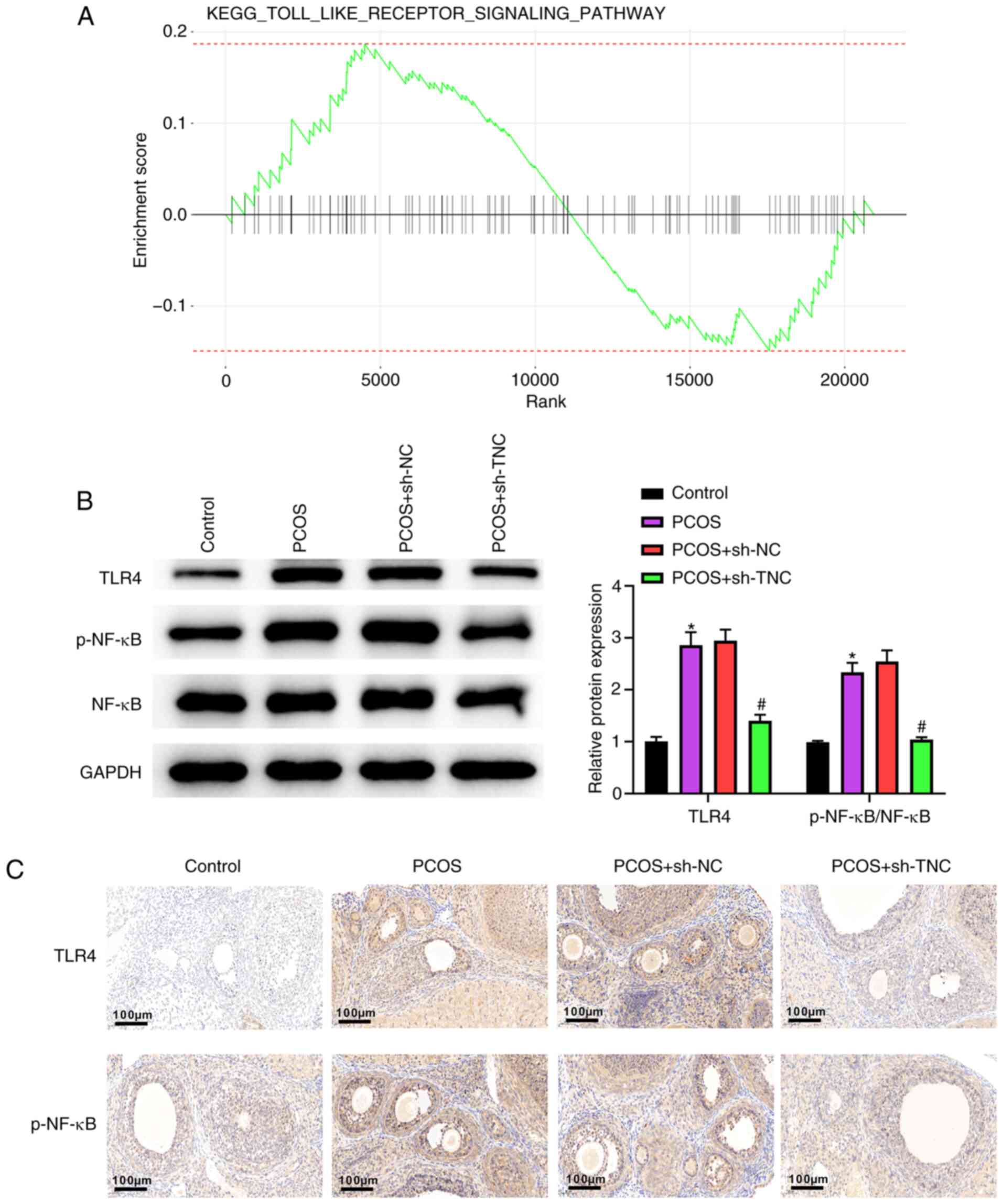
As shown in Fig. 4A, Kyoto Encyclopedia of Genes and Genomes analysis demonstrated that TNC inhibited the TLR pathway. Compared with those of the control group rats, TLR4 and p-NF-κB levels were elevated in PCOS group rats (Fig. 4B). TLR4 and p-NF-κB levels in PCOS + sh-TNC group rats were lower than those in PCOS + sh-NC group rats (Fig. 4B). The results of immunohistochemistry further confirmed this observation (Fig. 4C). All results suggested that knockdown of TNC could inhibit the activation of the TLR4/NF-κB signaling pathway in PCOS rats.
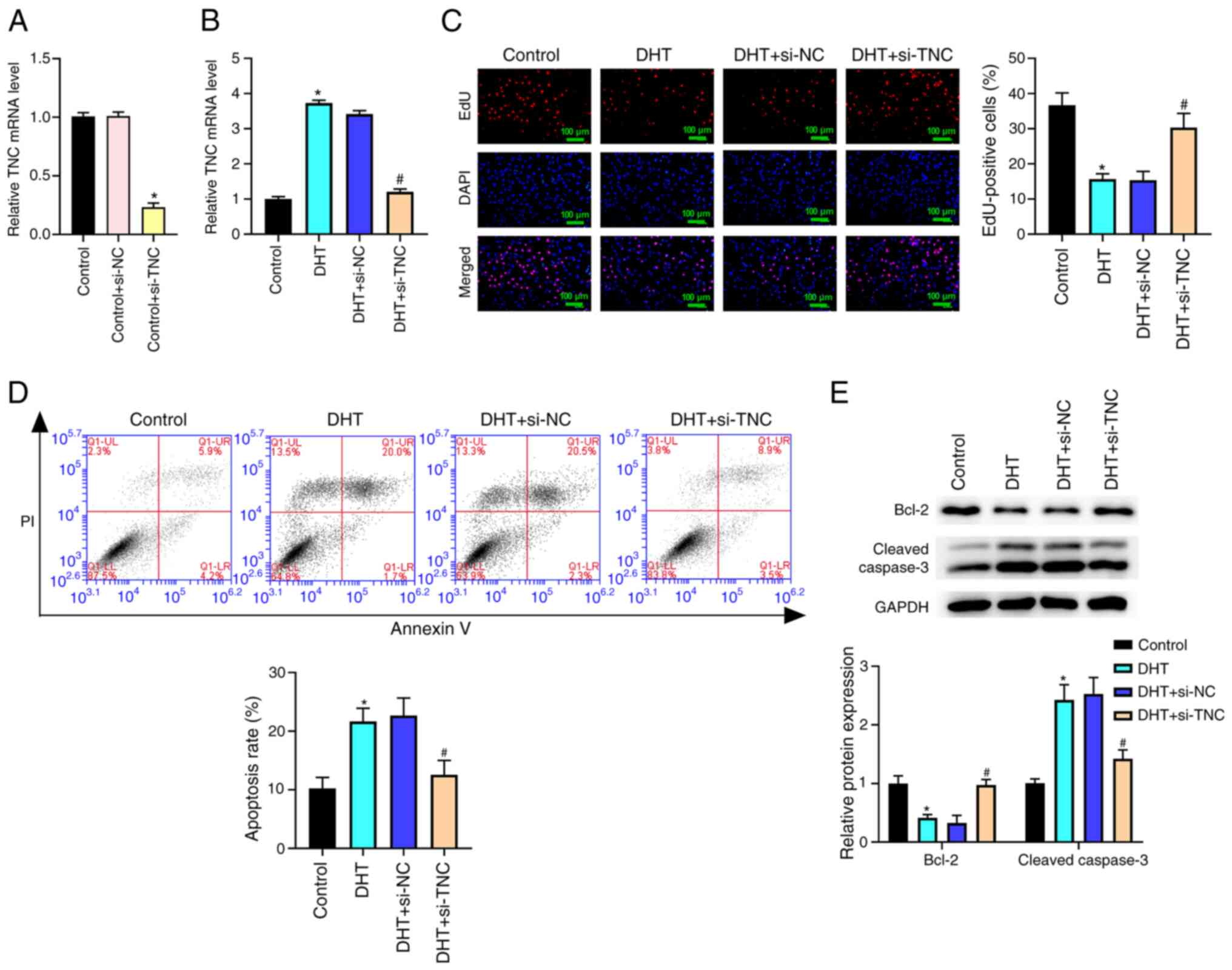
Knockdown of TNC reverses the reduced cell proliferation and the elevated cell apoptosis in KGN cells induced by DHT in vitro
As shown in Fig. 5A, TNC mRNA expression was significantly decreased in the control + si-TNC group compared with the control + si-NC group. The results in Fig. 5B demonstrated that the TNC level was higher in the DHT group than the control group. Compared with that in the DHT + si-NC group, the TNC level was significantly reduced in the DHT + si-TNC group (Fig. 5B). Additionally, the results of the EdU assay and flow cytometry demonstrated that TNC silencing notably reversed the DHT-induced reduction in cell proliferation and increase in apoptosis (Fig. 5C and D). In addition, TNC silencing significantly reversed the DHT-induced decrease in Bcl-2 expression and increase in cleaved caspase-3 expression in KGN cells (Fig. 5E).
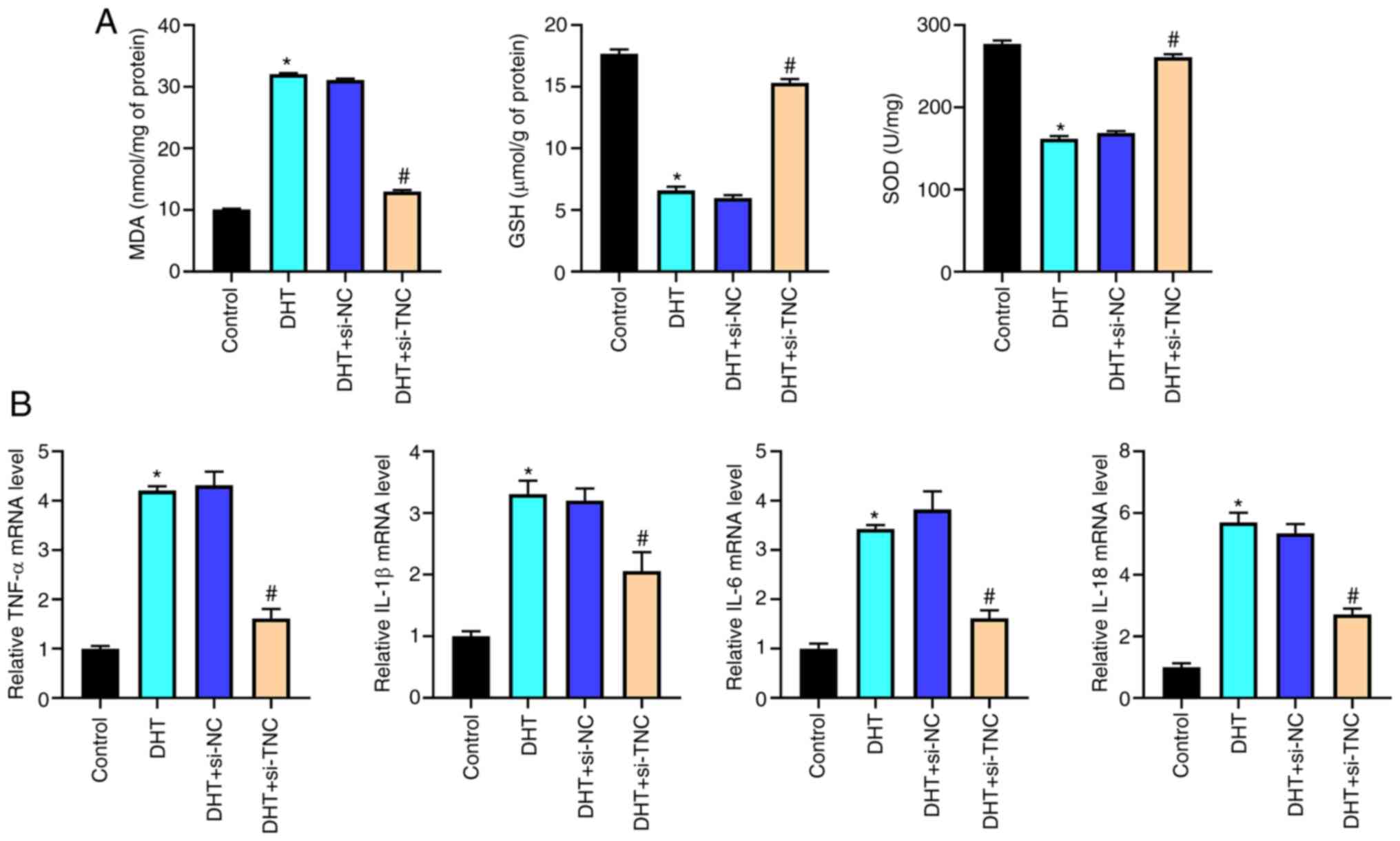
Knockdown of TNC alleviates the oxidative stress and inflammatory response in KGN cells induced by DHT in vitro
Elevated MDA levels, and downregulated GSH and SOD levels were observed in the DHT group compared with the control group; these phenotypes were reversed by silencing of TNC (Fig. 6A). In addition, the silencing of TNC significantly attenuated the increased expression levels of TNF-α, IL-1β, IL-6 and IL-18 in KGN cells induced by DHT (Fig. 6B). Together, these data indicated that knockdown of TNC could alleviate the DHT-induced oxidative stress and inflammatory response in KGN cells.
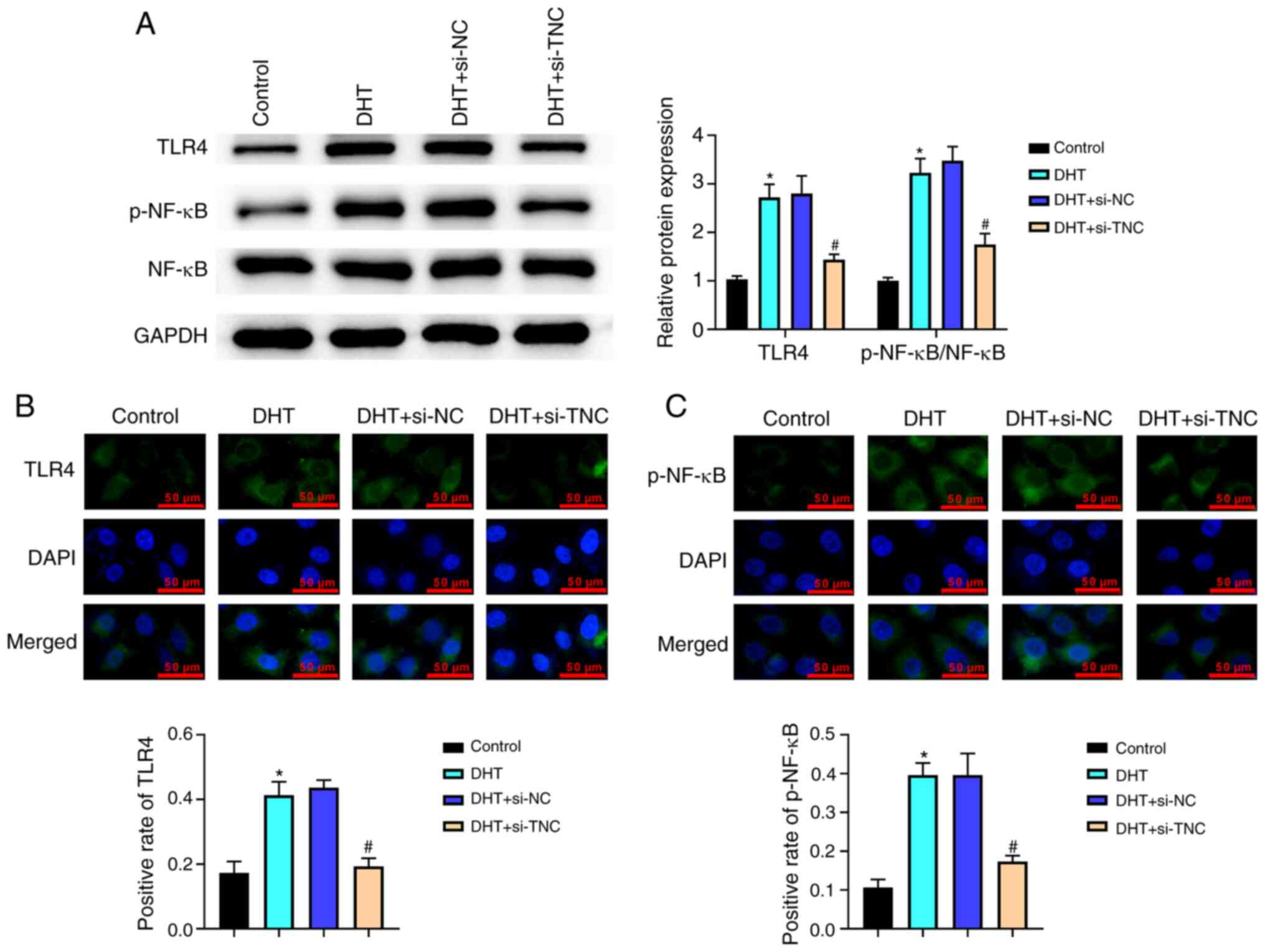
Knockdown of TNC inhibits the activation of the TLR4/NF-κB signaling pathway in DHT-treated KGN cells in vitro
As shown in Fig. 7A, the levels of TLR4 and p-NF-κB were significantly increased in the DHT group compared with the control group. When compared with those in the DHT + si-NC group, the levels of TLR4 and p-NF-κB were significantly decreased in the DHT + si-TNC group (Fig. 7A). This observation was also confirmed by immunofluorescence (Fig. 7B and C). Overall, knockdown of TNC could inhibit the activation of the TLR4/NF-κB signaling pathway in DHT-treated KGN cells.
Discussion
PCOS is a complex reproductive hormonal imbalance disease in women, with an incidence rate of 5–20% worldwide, which causes a high rate of infertility (2,32). Currently, in vitro fertilization and embryo transfer are common treatments for patients with failed pregnancies using ovulation induction (33). However, this places heavy physical, mental and financial burdens on patients. Thus, it is important to identify novel diagnostic and therapeutic biomarkers for PCOS. The present study revealed that knockdown of TNC could ameliorate PCOS in rat and cell models by inhibiting cell apoptosis, oxidative stress and inflammation, and attenuating IR via suppression of the activation of the TLR4/NF-κB signaling pathway.
PCOS is generally considered to be closely related to hyperandrogenemia, IR, chronic inflammation and oxidative stress (5,34,35). IR has been found in 75% of patients with PCOS (4). In the present study, knockdown of TNC significantly reduced the HOMA-IR index in PCOS rats. Additionally, knockdown of TNC markedly reversed the elevation of LH, testosterone and estradiol levels induced by DHEA. Oxidative stress has been reported to serve an important role in PCOS (36). Circulating oxidative biomarkers are increased in women with PCOS compared with controls (5). The present study found that knockdown of TNC reversed the elevation of MDA levels and reduction of GSH and SOD levels in PCOS rats and DHT-induced KGN cells. An increasing number of studies have demonstrated that chronic inflammation contributes to the progression of PCOS (37–39). The principal markers of the inflammatory state include C-reactive protein, TNF-α, IL-1β, IL-6 and IL-18 (32). In the present study, knockdown of TNC significantly reversed the increase in expression of pro-inflammatory cytokines in rat and cellular PCOS models. These data suggested that TNC may be a promising novel target for the development of targeted therapies for PCOS.
There are some studies on the roles of TNC in the treatment of multiple diseases via regulation of cell apoptosis, oxidative stress and inflammatory responses (16,40–43). Knockdown of TNC has been reported to suppress inflammation and apoptosis via the PI3K/Akt/NF-κB signaling pathway in subarachnoid hemorrhage (44). TNC enhances the pro-inflammatory phenotype of macrophages (45). Yonebayashi et al (46) demonstrated that TNC upregulation promoted the levels of proinflammatory cytokines/chemokines in mouse hearts and increased mortality rates during the acute stage after myocardial infarction. Furthermore, increased expression of TNC is considered to induce inflammatory mediators and accelerate matrix degradation in osteoarthritis cartilage (47). These aforementioned observations are consistent with the results of the present experiments, in which knockdown of TNC was able to alleviate inflammation and inhibit apoptosis and oxidative stress in PCOS.
An increasing number of studies have reported that multiple signaling cascades, such as TGF-β/Smad-3/4, TLR4/NF-κB, platelet derived growth factor subunit B (PDGFB)/phosphoinositide 3-kinase/Akt and PDGFB/mitogen activated kinase-like protein, could regulate TNC expression (45,48,49). TLR4 has been verified to serve a vital role in proinflammatory signaling and may contribute to the progression of PCOS (50,51). TNC functions as a damage-associated molecular pattern and is one of the ligands of TLR4 (52,53). TLR4 recruits a sequence of downstream adaptors and further activates downstream mediators, including NF-κB (54). Consequently, numerous pro-inflammatory cytokines are produced (55,56). A number of studies have confirmed that TLR4/NF-κB signaling activation could promote the development of PCOS (57,58). For example, cryptotanshinone could alleviate PCOS in rats by modulating the high mobility group box 1/TLR4/NF-κB signaling pathway (59). Sitagliptin combined with rosiglitazone inhibited TLR4/NF-κB signaling, thereby repressing autophagy and inflammation in PCOS rats (60). Notably, TNC exerts pro-inflammatory effects via the activation of TLR4 (61). In the present study, TNC knockdown could inhibit the activation of the TLR4/NF-κB signaling pathway in PCOS rat and cell models.
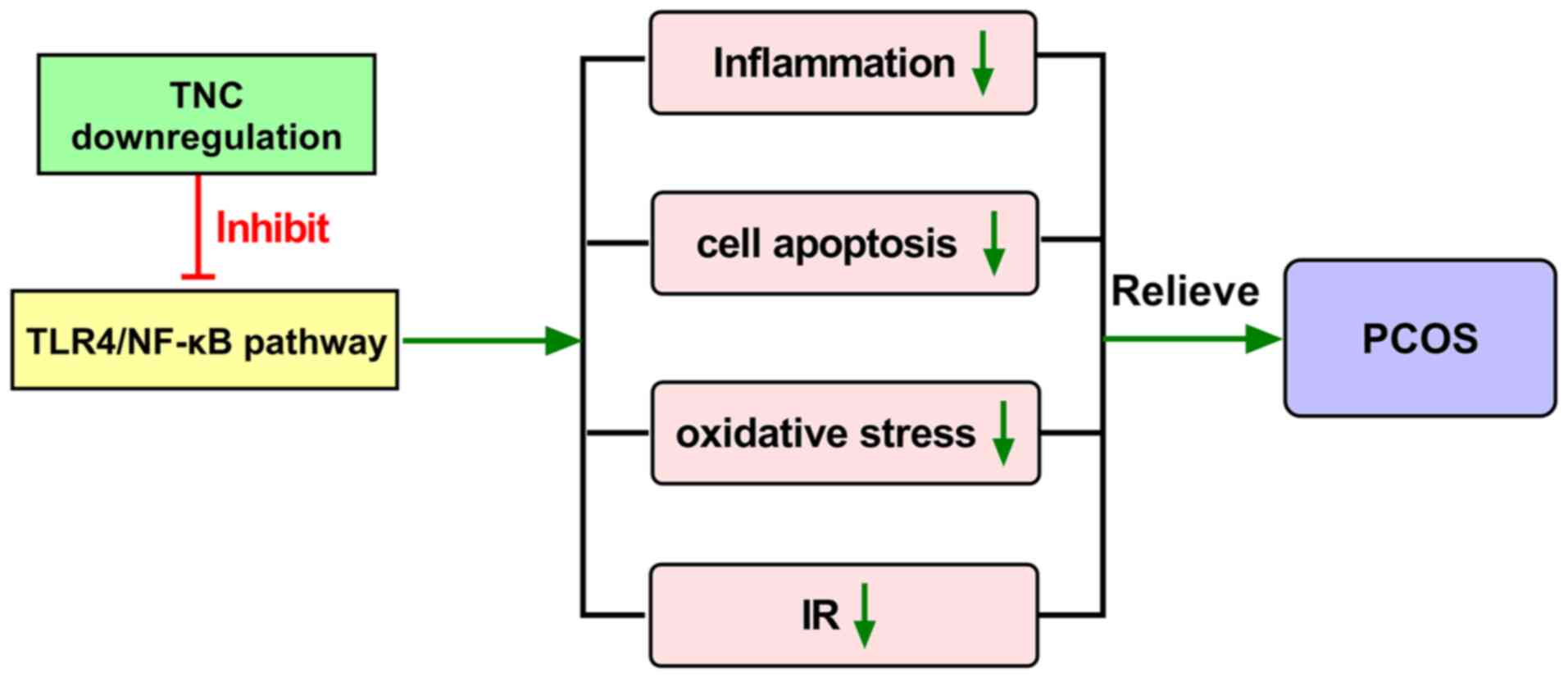
In conclusion, the present study revealed that knockdown of TNC ameliorated PCOS in rat and cell models by inhibiting cell apoptosis, oxidative stress and inflammation, and attenuating IR via suppression of the activation of the TLR4/NF-κB signaling pathway (Fig. 8). These findings may help reveal a novel role of TNC in the development of PCOS. The present study has some limitations. Firstly, further studies are required to identify other potential mechanisms of TNC that are involved in PCOS. Secondly, TNC expression in clinical samples, and the relationship between TNC expression in PCOS and clinical features, including infertility, should be explored and analyzed in future experiments. Additionally, whether TNC may promote PCOS development in humans by mediating the TLR4/NF-κB signaling pathway needs to be further verified.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science Foundation of Shandong Province (grant no. ZR2016HL04), and Science and Technology Innovation Development Project of Taian City (grant no. 2020NS266).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
HWu, MY and CY performed the experiments. ML, HWa and WZ provided technical support and analyzed the data. HWu contributed to manuscript preparation. HWu and WZ confirmed the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The experimental protocol of the present study was performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Ethical Committee of The Second Affiliated Hospital of Shandong First Medical University (approval no. 2021-128; Taian, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Kumariya S, Ubba V, Jha RK and Gayen JR: Autophagy in ovary and polycystic ovary syndrome: Role, dispute and future perspective. Autophagy. 17:2706–2733. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ and Yildiz BO: Polycystic ovary syndrome. Nat Rev Dis Primers. 2:160572016. View Article : Google Scholar : PubMed/NCBI | |
|
Siddiqui S, Mateen S, Ahmad R and Moin S: A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J Assist Reprod Genet. 39:2439–2473. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Moghetti P and Tosi F: Insulin resistance and PCOS: Chicken or egg? J Endocrinol Invest. 44:233–244. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Murri M, Luque-Ramírez M, Insenser M, Ojeda-Ojeda M and Escobar-Morreale HF: Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Hum Reprod Update. 19:268–288. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang XL, Tai H, Xiao XS, Zhang SY, Cui SC, Qi SB, Hu DD, Zhang LN, Kuang JS, Meng XS and Li SM: Cangfudaotan decoction inhibits mitochondria-dependent apoptosis of granulosa cells in rats with polycystic ovarian syndrome. Front Endocrinol (Lausanne). 13:9621542022. View Article : Google Scholar : PubMed/NCBI | |
|
Norman RJ, Dewailly D, Legro RS and Hickey TE: Polycystic ovary syndrome. Lancet. 370:685–697. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Lu Z, Chen C, Gao Y, Li Y, Zhao X, Zhang H, Wei Q, Zeng X, Li Y and Wan M: Screening target genes for the treatment of PCOS via analysis of single-cell sequencing data. Ann Med. 54:2975–2989. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Liu X, Sun C, Zou K, Li C, Chen X, Gu H, Zhou Z, Yang Z, Tu Y, Qin N, et al: Novel PGK1 determines SKP2-dependent AR stability and reprograms granular cell glucose metabolism facilitating ovulation dysfunction. EBioMedicine. 61:1030582020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z, Dong H, Yang L, Yi P, Wang Q and Huang D: The role of FDX1 in granulosa cell of polycystic ovary syndrome (PCOS). BMC Endocr Disord. 21:1192021. View Article : Google Scholar : PubMed/NCBI | |
|
Imanaka-Yoshida K and Aoki H: Tenascin-C and mechanotransduction in the development and diseases of cardiovascular system. Front Physiol. 5:2832014. View Article : Google Scholar : PubMed/NCBI | |
|
Faissner A, Roll L and Theocharidis U: Tenascin-C in the matrisome of neural stem and progenitor cells. Mol Cell Neurosci. 81:22–31. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Chen S, Fu H, Wu S, Zhu W, Liao J, Hong X, Miao J, Luo C, Wang Y, Hou FF, et al: Tenascin-C protects against acute kidney injury by recruiting Wnt ligands. Kidney Int. 95:62–74. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Song W and Qiu N: MiR-495-3p depletion contributes to myocardial ischemia/reperfusion injury in cardiomyocytes by targeting TNC. Regen Ther. 21:380–388. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Ishizaki J, Takemori A, Suemori K, Matsumoto T, Akita Y, Sada KE, Yuzawa Y, Amano K, Takasaki Y, Harigai M, et al: Targeted proteomics reveals promising biomarkers of disease activity and organ involvement in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Res Ther. 19:2182017. View Article : Google Scholar : PubMed/NCBI | |
|
Cheng H, Li L, Xue J, Ma J and Ge J: TNC accelerates hypoxia-induced cardiac injury in a METTL3-dependent manner. Genes (Basel). 14:5912023. View Article : Google Scholar : PubMed/NCBI | |
|
Li Z, Chen S, Cui H, Li X, Chen D, Hao W, Wang J, Li Z, Zheng Z, Zhang Z and Liu H: Tenascin-C-mediated suppression of extracellular matrix adhesion force promotes entheseal new bone formation through activation of Hippo signalling in ankylosing spondylitis. Ann Rheum Dis. 80:891–902. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Imanaka-Yoshida K, Tawara I and Yoshida T: Tenascin-C in cardiac disease: A sophisticated controller of inflammation, repair, and fibrosis. Am J Physiol Cell Physiol. 319:C781–C796. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Dumesic DA, Phan JD, Leung KL, Grogan TR, Ding X, Li X, Hoyos LR, Abbott DH and Chazenbalk GD: Adipose insulin resistance in normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 104:2171–2183. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Xu A, Fan Y, Liu S, Sheng L, Sun Y and Yang H: GIMAP7 induces oxidative stress and apoptosis of ovarian granulosa cells in polycystic ovary syndrome by inhibiting sonic hedgehog signalling pathway. J Ovarian Res. 15:1412022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Hu M, Jia W, Liu G, Zhang J, Wang B, Li J, Cui P, Li X, Lager S, et al: Hyperandrogenism and insulin resistance modulate gravid uterine and placental ferroptosis in PCOS-like rats. J Endocrinol. 246:247–263. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Dăneasă A, Cucolaş C, Lenghel LM, Olteanu D, Orăsan R and Filip GA: Letrozole vs estradiol valerate induced PCOS in rats: Glycemic, oxidative and inflammatory status assessment. Reproduction. 151:401–409. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Feldmann RE Jr, Maurer MH, Hunzinger C, Lewicka S, Buergers HF, Kalenka A, Hinkelbein J, Broemme JO, Seidler GH, Martin E and Plaschke K: Reduction in rat phosphatidylethanolamine binding protein-1 (PEBP1) after chronic corticosterone treatment may be paralleled by cognitive impairment: A first study. Stress. 11:134–147. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Van Way CW, Dhar A, Reddy R, Evans L, Wogahn B and Helling TS: Changes in adenine nucleotides during hemorrhagic shock and reperfusion. J Surg Res. 66:159–166. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Weksler B, Schneider A, Ng B and Burt M: Isolated single lung perfusion in the rat: An experimental model. J Appl Physiol (1985). 74:2736–2739. 1993. View Article : Google Scholar : PubMed/NCBI | |
|
Edge D, Shortt CM, Johns EJ, Gobbo OL, Markos F, Abdulla MH and Barry EF: Assessment of renal function in the anaesthetised rat following injection of superparamagnetic iron oxide nanoparticles. Can J Physiol Pharmacol. 95:443–446. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Strohmaier CA, Motloch K, Runge C, Trost A, Bogner B, Kaser-Eichberger A, Schrödl F, Lenzhofer M and Reitsamer HA: Retinal vessel diameter responses to central electrical stimulation in the rat: Effect of nitric oxide synthase inhibition. Invest Ophthalmol Vis Sci. 57:4553–4557. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Clifford PS, Ferguson BS, Jasperse JL and Hill MA: Arteriolar vasodilation involves actin depolymerization. Am J Physiol Heart Circ Physiol. 315:H423–H428. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Huang Y and Zhang X: Luteolin alleviates polycystic ovary syndrome in rats by resolving insulin resistance and oxidative stress. Am J Physiol Endocrinol Metab. 320:E1085–E1092. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Corbould A, Kim YB, Youngren JF, Pender C, Kahn BB, Lee A and Dunaif A: Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab. 288:E1047–E1054. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
de Medeiros SF, de Medeiros MAS, Santos NS, Barbosa BB and Yamamoto MMW: Combined oral contraceptive effects on low-grade chronic inflammatory mediators in women with polycystic ovary syndrome: A systematic review and meta-analysis. Int J Inflam. 2018:95915092018.PubMed/NCBI | |
|
Zheng X, Guo W, Zeng L, Zheng D, Yang S, Wang L, Wang R, Mol BW, Li R and Qiao J: Live birth after in vitro maturation versus standard in vitro fertilisation for women with polycystic ovary syndrome: Protocol for a non-inferiority randomised clinical trial. BMJ Open. 10:e0353342020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang T, Sha L, Li Y, Zhu L, Wang Z, Li K, Lu H, Bao T, Guo L, Zhang X and Wang H: Dietary α-linolenic acid-rich flaxseed oil exerts beneficial effects on polycystic ovary syndrome through sex steroid hormones-microbiota-inflammation axis in rats. Front Endocrinol (Lausanne). 11:2842020. View Article : Google Scholar : PubMed/NCBI | |
|
Diamanti-Kandarakis E and Dunaif A: Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr Rev. 33:981–1030. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
González F, Considine RV, Abdelhadi OA and Acton AJ: Oxidative stress in response to saturated fat ingestion is linked to insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 104:5360–5371. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Wang D, Wang T, Wang R, Zhang X, Wang L, Xiang Z, Zhuang L, Shen S, Wang H, Gao Q and Wang Y: Suppression of p66Shc prevents hyperandrogenism-induced ovarian oxidative stress and fibrosis. J Transl Med. 18:842020. View Article : Google Scholar : PubMed/NCBI | |
|
Shorakae S, Ranasinha S, Abell S, Lambert G, Lambert E, de Courten B and Teede H: Inter-related effects of insulin resistance, hyperandrogenism, sympathetic dysfunction and chronic inflammation in PCOS. Clin Endocrinol (Oxf). 89:628–633. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Liu M, Gao J, Zhang Y, Li P, Wang H, Ren X and Li C: Serum levels of TSP-1, NF-κB and TGF-β1 in polycystic ovarian syndrome (PCOS) patients in northern China suggest PCOS is associated with chronic inflammation. Clin Endocrinol (Oxf). 83:913–922. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Chen H, Yang J and Tan Z: Upregulation of microRNA-9-5p inhibits apoptosis of chondrocytes through downregulating Tnc in mice with osteoarthritis following tibial plateau fracture. J Cell Physiol. 234:23326–23336. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Shiba M, Fujimoto M, Imanaka-Yoshida K, Yoshida T, Taki W and Suzuki H: Tenascin-C causes neuronal apoptosis after subarachnoid hemorrhage in rats. Transl Stroke Res. 5:238–247. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Y, Ma XY, Han JY, Yang M, Lv C, Shao Y, Wang YL, Kang JY and Wang QY: Metformin regulates inflammation and fibrosis in diabetic kidney disease through TNC/TLR4/NF-κB/miR-155-5p inflammatory loop. World J Diabetes. 12:19–46. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Karataş Z, Baysal T, Sap F, Altın H and Çiçekler H: The role of tenascin-C and oxidative stress in rheumatic and congenital heart valve diseases: An observational study. Anadolu Kardiyol Derg. 13:350–356. 2013.PubMed/NCBI | |
|
Tong X, Zhang J and Shen M: Silencing of tenascin-C inhibited inflammation and apoptosis via PI3K/Akt/NF-κB signaling pathway in subarachnoid hemorrhage cell model. J Stroke Cerebrovasc Dis. 29:1044852020. View Article : Google Scholar : PubMed/NCBI | |
|
Imanaka-Yoshida K: Tenascin-C in heart diseases-the role of inflammation. Int J Mol Sci. 22:58282021. View Article : Google Scholar : PubMed/NCBI | |
|
Yonebayashi S, Tajiri K, Hara M, Saito H, Suzuki N, Sakai S, Kimura T, Sato A, Sekimoto A, Fujita S, et al: Generation of transgenic mice that conditionally overexpress tenascin-C. Front Immunol. 12:6205412021. View Article : Google Scholar : PubMed/NCBI | |
|
Patel L, Sun W, Glasson SS, Morris EA, Flannery CR and Chockalingam PS: Tenascin-C induces inflammatory mediators and matrix degradation in osteoarthritic cartilage. BMC Musculoskelet Disord. 12:1642011. View Article : Google Scholar : PubMed/NCBI | |
|
Chiovaro F, Chiquet-Ehrismann R and Chiquet M: Transcriptional regulation of tenascin genes. Cell Adh Migr. 9:34–47. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Midwood KS, Chiquet M, Tucker RP and Orend G: Tenascin-C at a glance. J Cell Sci. 129:4321–4327. 2016.PubMed/NCBI | |
|
Wang Y, He J and Yang J: Eicosapentaenoic acid improves polycystic ovary syndrome in rats via sterol regulatory element-binding protein 1 (SREBP-1)/toll-like receptor 4 (TLR4) pathway. Med Sci Monit. 24:2091–2097. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Shen HR, Xu X and Li XL: Berberine exerts a protective effect on rats with polycystic ovary syndrome by inhibiting the inflammatory response and cell apoptosis. Reprod Biol Endocrinol. 19:32021. View Article : Google Scholar : PubMed/NCBI | |
|
Turner NA: Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs). J Mol Cell Cardiol. 94:189–200. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Marzeda AM and Midwood KS: Internal affairs: Tenascin-C as a clinically relevant, endogenous driver of innate immunity. J Histochem Cytochem. 66:289–304. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Liu L, Li YH, Niu YB, Sun Y, Guo ZJ, Li Q, Li C, Feng J, Cao SS and Mei QB: An apple oligogalactan prevents against inflammation and carcinogenesis by targeting LPS/TLR4/NF-κB pathway in a mouse model of colitis-associated colon cancer. Carcinogenesis. 31:1822–1832. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z, Zhai D, Zhang D, Bai L, Yao R, Yu J, Cheng W and Yu C: Quercetin decreases insulin resistance in a polycystic ovary syndrome rat model by improving inflammatory microenvironment. Reprod Sci. 24:682–690. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Bhaskar S, Shalini V and Helen A: Quercetin regulates oxidized LDL induced inflammatory changes in human PBMCs by modulating the TLR-NF-κB signaling pathway. Immunobiology. 216:367–373. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Hu M, Zhang Y, Li X, Cui P, Sferruzzi-Perri AN, Brännström M, Shao LR and Billig H: TLR4-associated IRF-7 and NFκB signaling act as a molecular link between androgen and metformin activities and cytokine synthesis in the PCOS endometrium. J Clin Endocrinol Metab. 106:1022–1040. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Jin W, Wang C, Cui M, Wang M, Fu B, Sun L and Chen X: Inhibitory effect of bushen huoxue formula against dehydroepiandrosterone-induced inflammation in granulosa cells through TLR4/NF-κB signaling pathway. Pak J Pharm Sci. 35:701–710. 2022.PubMed/NCBI | |
|
Yang Y, Yang L, Qi C, Hu G, Wang L, Sun Z and Ni X: Cryptotanshinone alleviates polycystic ovary syndrome in rats by regulating the HMGB1/TLR4/NF-κB signaling pathway. Mol Med Rep. 22:3851–3861. 2020.PubMed/NCBI | |
|
Ren Y, Ye Y, Xuan F, Chen A, Jin R, Zhou W and Lu J: The effect of sitagliptin combined with rosiglitazone on autophagy and inflammation in polycystic ovary syndrome by regulating PI3K/AKT/mTOR and TLR4/NF-κB pathway. Reprod Biol. 23:1007632023. View Article : Google Scholar : PubMed/NCBI | |
|
Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, Drexler S, Sofat N, Kashiwagi M, Orend G, et al: Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 15:774–780. 2009. View Article : Google Scholar : PubMed/NCBI |