Immune analysis of urine and plasma samples from patients with clear cell renal cell carcinoma
- Authors:
- Published online on: April 25, 2024 https://doi.org/10.3892/ol.2024.14414
- Article Number: 281
-
Copyright: © Vargová et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Renal cell carcinoma (RCC) is predominantly (~75% worldwide) represented by the clear cell variant, which is known as clear cell RCC (ccRCC) (1). In 2020, RCC was ranked 14th in terms of global cancer incidence, and was ranked 3rd among urological malignancies in terms of incidence and mortality (2). The global incidence of RCC has been increasing by 2% per year over the last two decades (3), and it is estimated that this trend will continue in the future (4). Since this cancer type exhibits silent progression and late manifestation, the majority of cases are identified incidentally (5,6), and patients with a metastatic form of RCC represent 35–50% of new diagnoses (7). Despite the new pharmacological treatment possibilities, these patients have markedly low survival rates; the 5-year survival rate for patients in the advanced stages of ccRCC is estimated to be <12% (4). By contrast, ccRCC is largely curable when it is diagnosed at the early stages (8). Therefore, one of the main priorities of RCC research is to identify potential biomarkers suitable for early non-invasive or minimally invasive detection of ccRCC and/or follow-up of patients after surgery (8,9).
The tumor microenvironment (TME) of RCC is known to be densely infiltrated by various immune cells (10,11). Leukocytes are the main source of immune signaling molecules, namely cytokines and chemokines, which serve an important role in cancer-related processes (12,13). Increased levels of these inflammatory mediators have previously been detected in tissue and serum from patients with RCC (14–17). In the present study, a complex multiplex immune analysis was performed, including the detection of 27 biologically relevant cytokines in urine and plasma samples from patients diagnosed with ccRCC, and their diagnostic significance was evaluated.
Patients and methods
Study dataset
A total of 34 patients (age range, 46–86 years) with histologically confirmed ccRCC were recruited over a 3-year period (June 2021 to March 2023) from the Department of Urology, University Hospital Martin (Martin, Slovakia) for the current study. All patients were recommended surgical treatment (total or partial nephrectomy) and underwent clinical and histological classification according to the Tumor-Node-Metastasis classification system (18) and the World Health Organization/International Society of Urological Pathology four-tier classification system of tumor grade (19). The clinicopathological characteristics of the patients are shown in Table I. Patients with chronic inflammation of the urogenital system, systemic autoimmune disease, or endocrine, severe cardiovascular or rheumatological disease, or those undergoing anti-inflammatory or immunosuppressive therapy were excluded from the study.
Urine samples were obtained from all patients on the day of surgery prior to removal of the tumor (preoperative urine; U0), and from 23 patients also on day 3 post-surgery (postoperative day 3 urine; U3). Peripheral venous blood (6 ml) was obtained from 14 patients prior to surgery. Urine samples were collected into sterile polypropylene tubes, whereas blood samples were collected into EDTA tubes, transported promptly to the laboratory and immediately centrifuged (877 × g for 10 min at 4°C) to separate plasma. Both urine and plasma samples were aliquoted and kept frozen at −80°C until further analysis.
A total of 9 control subjects provided negative control urine (UC) samples, and an additional 7 control subjects provided negative control samples of blood. These control groups consisted of individuals without any oncological disease of the urogenital system or any systemic inflammatory disease and were recruited from healthy individuals undergoing preventive medical examination at the Department of Urology, University Hospital Martin. The distribution of the subjects in the different study groups according to their sex and mean age is shown in Table II. Each participant in the study provided written informed consent prior to the start of the study. Institutional ethics approval from the Ethics Committee of the Jessenius Faculty of Medicine of Martin, Comenius University in Bratislava (approval no. 19/2019; Martin, Slovakia) was obtained for the present study.
Evaluation of urinary and plasma cytokines
Cytokine concentrations were quantified in plasma and urine samples using the standard 27-Plex BioPlex™ Human Cytokine Assay (cat. no. M500KCAF0Y; Bio-Rad Laboratories, Inc.). The screening panel consisted of 27 cell signaling molecules involved in critical biological and pathological events and signaling pathways, namely basic fibroblastic factor (bFGF), chemokine (C-C motif) ligand (CCL)11 (also known as eotaxin), granulocyte-macrophage colony stimulating factor (GM-CSF), granulocyte colony stimulating factor (G-CSF), IFN-γ, IL-1β, IL-1 receptor antagonist (IL-1Ra), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8 [the latter also known as C-X-C motif chemokine ligand (CXCL)8], IL-9, IL-10, IL-12p70, IL-13, IL-15 and IL-17A, IFN-γ-induced protein 10 (also known as CXCL10), monocyte chemoattractant protein 1 (also known as CCL2), macrophage inflammatory proteins 1α and β (also known as CCL3 and CCL4, respectively), platelet-derived growth factor-BB (PDGF-BB), regulated upon activation, normal T cell expressed and secreted (also known as CCL5), TNF-α and vascular endothelial growth factor (VEGF). The assay was carried out on 50 µl samples and was performed according to the manufacturer's instructions. Visualization of target molecules was performed using the Bio-Plex 200 System reader (Bio-Rad Laboratories, Inc.) and the experimental data were processed by Bio-Plex Manager™ software version 6.1 (Bio-Rad Laboratories, Inc.) using a 5-parameter logistic curve. The levels of cytokines were presented as median fluorescence intensity and expressed in pg/ml.
Hemoglobinometry
All urine samples were tested for the presence of hemoglobin (Hgb) in order to exclude any false positives (namely cytokines originating in blood circulation). A quantitative, multiparameter automated hematology analyzer (DxH 900; Beckman Coulter, Inc.) was used for this purpose. This instrument allowed for time-efficient measurement of cell blood count and volume, and determination of the level of Hgb in body fluids. Quantification of Hgb was performed photometrically at 525 nm and according to the manufacturer's instructions. The signal from the sample was then compared to a Hgb blank (commercially available), which was used as a reference sample. Hgb presence was positive in 6 preoperative and 2 postoperative urine samples. Therefore, these samples were excluded from further analysis.
Statistical analysis
Initially, all of the data were subjected to a Shapiro-Wilk normality test. Since urinary cytokines did not fulfill the criteria for normal distribution, they were logarithmically transformed. Plasma cytokines were used in further analyses on the original scale (pg/ml). Gross outliers were removed using ROUT method, and sample means with standard deviations were calculated. The transformed and cleaned data were visualized in the form of stacked bar charts and column graphs, while an individual values plot was presented in Fig. S1. In addition, heatmaps were created using MetaboAnalyst, a free online platform for metabolomic data analysis (https://www.metaboanalyst.ca/). Unpaired Student's t-tests were used to test the null hypothesis on the equality of means of different subpopulations of study subjects, namely plasma of cases versus plasma of controls, U0 versus UC, and U3 versus UC. Paired t-test was used to test the null hypothesis on the equality of means of matched U0 and U3 samples. The effect size quantified by Cohen's D and statistical power of the significant findings were calculated using G*Power software 3.1 (20). U0 samples (n=28) were stratified according to tumor grade into two groups: A low-grade (LG) group consisting of G1-2 tumors, and a high-grade (HG) group consisting of G3-4 tumors. One-way ANOVA was used to test the equality of population means across three groups: LG, HG and controls. If the difference was significant, Tukey's post hoc test was performed. The correlation between plasma and preoperative urine samples of patients was evaluated by Pearson's correlation test. Analysis and visualization of the results were performed with GraphPad Prism software v. 8.0.1 (Dotmatics). P<0.05 was considered to indicate a statistically significant difference.
Results
Urinary cytokine profile of patients with ccRCC before and after nephrectomy
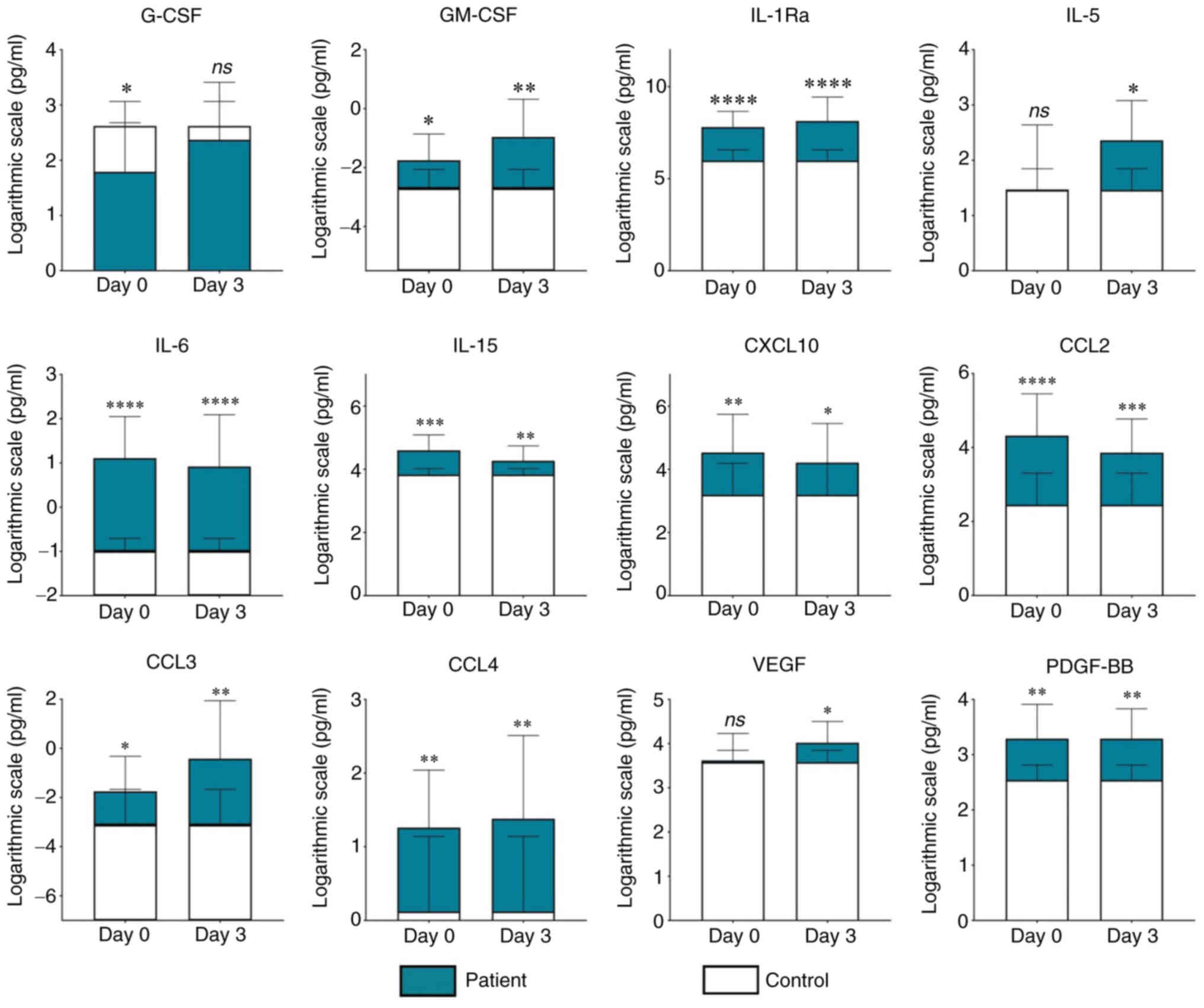
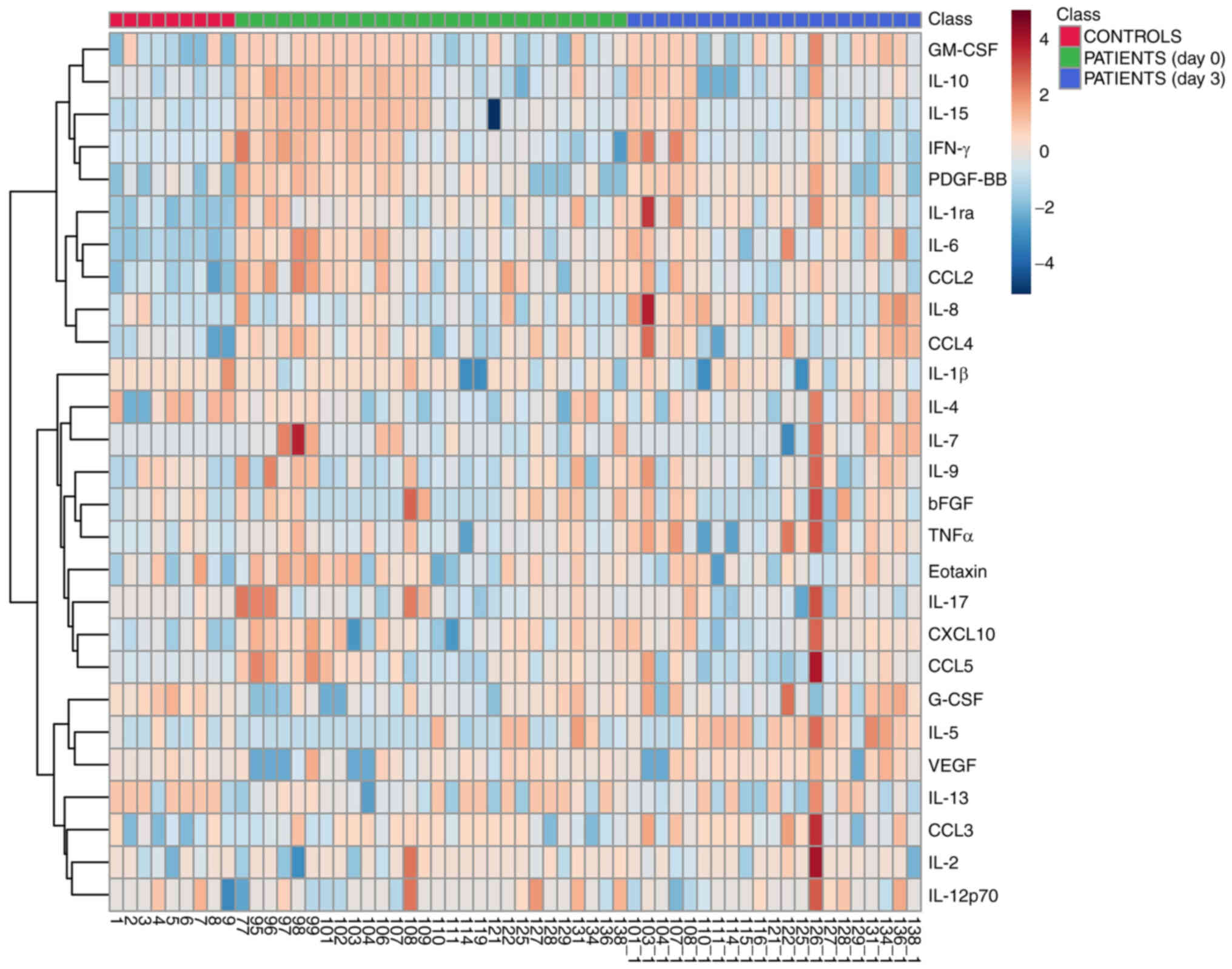
After excluding the samples with Hgb positivity, 28 U0 and 21 U3 samples from patients with ccRCC were compared with 9 UC samples. Statistical analysis revealed that both U0 and U3 samples contained significantly higher levels of IL-1Ra (U0 vs. UC, P<0.0001; U3 vs. UC, P<0.0001), IL-6 (U0 vs. UC, P<0.0001; U3 vs. UC, P<0.0001), IL-15 (U0 vs. UC, P<0.001; U3 vs. UC, P=0.01), CCL2 (U0 vs. UC, P<0.0001; U3 vs. UC, P<0.001), CCL3 (U0 vs. UC, P=0.04; U3 vs. UC, P=0.01), CCL4 (U0 vs. UC, P=0.002; U3 vs. UC, P=0.01), CXCL10 (U0 vs. UC, P=0.005; U3 vs. UC, P=0.04), GM-CSF (U0 vs. UC, P=0.02; U3 vs. UC, P=0.003) and PDGF-BB (U0 vs. UC, P=0.01; U3 vs. UC, P=0.007) compared with UC samples (Fig. 1). Additionally, VEGF and IL-5 were significantly increased in U3 (U3 vs. UC, P=0.02 and P=0.03, respectively) but not in U0 (Fig. 1). By contrast, G-CSF reached significantly higher levels in UC compared with U0 (P=0.01), but no significant difference in its concentration was observed between U3 and UC (Fig. 1). Table SI specifies the size of statistical power of all significant findings detected by this analysis. The heatmap in Fig. 2 provides a comprehensive graphical view of the levels of all the examined cytokines in UC, U0 and U3. In addition to the aforementioned analysis, additional investigations were performed: i) A pairwise comparison between preoperative and postoperative urine samples from patients (Fig. S1; Table SII), and ii) a comparison between 5 urine samples from patients (collected on average 10 months after surgery) and 9 controls (Fig. S2; Table SIII).
Urinary cytokine profiles of patients with ccRCC according to tumor grade
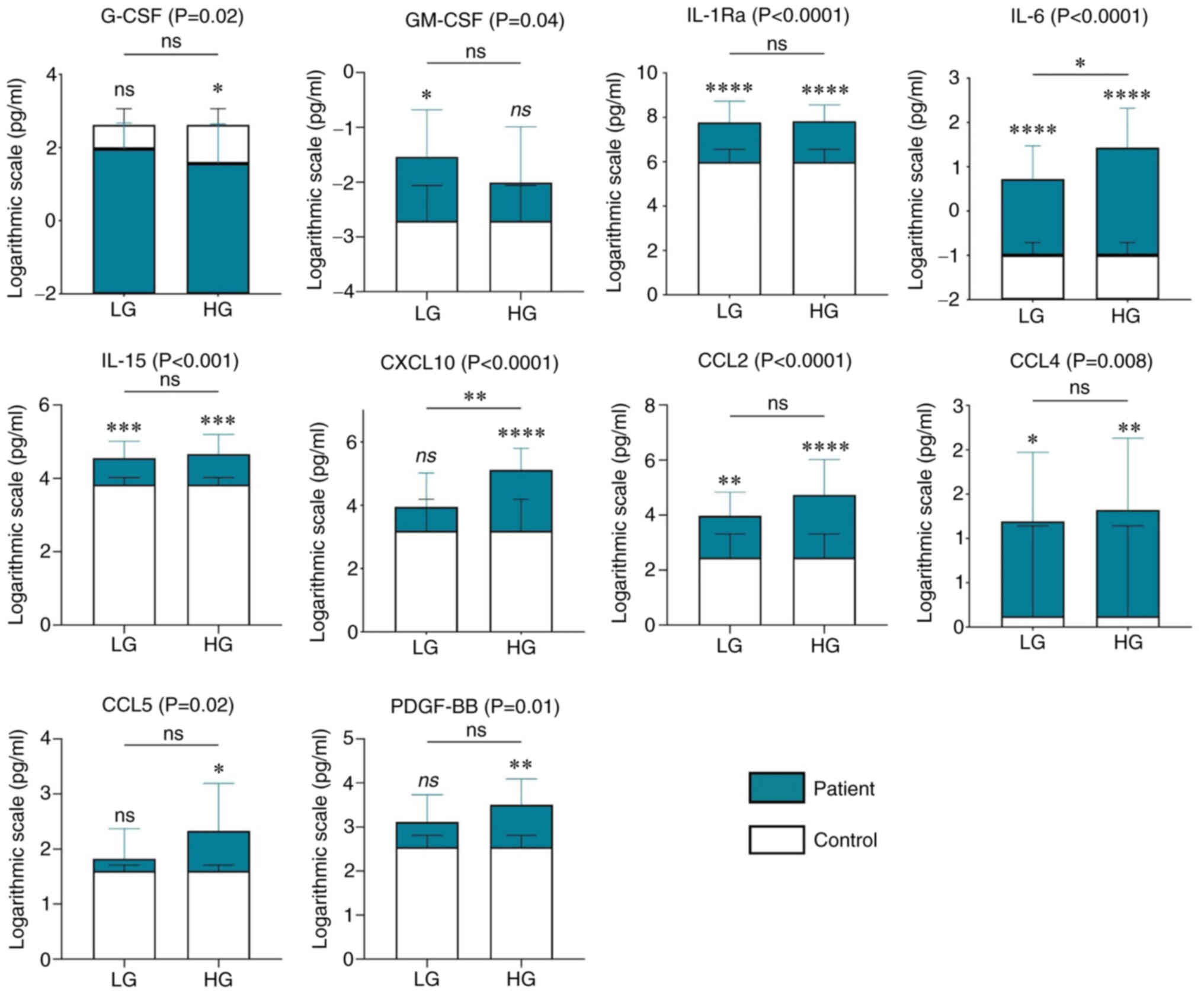
A comparative analysis between urine samples from patients with LG (n=15) and HG (n=13) ccRCC, and from controls (n=9) was performed. Significant differences between LG and control groups and/or HG and control groups were observed regarding the levels of G-CSF [adjusted P-value (Adj. P) of HG vs. C, 0.02], GM-CSF [Adj. P (LG vs. C), 0.03], IL-1Ra [Adj. P (LG vs. C), <0.0001; Adj. P (HG vs. C), <0.0001], IL-6 [Adj. P (LG vs. C), <0.0001; Adj. P (HG vs. C), <0.0001], IL-15 [Adj. P (LG vs. C), 0.001; Adj. P (HG vs. C), <0.001], CXCL10 [Adj. P (HG vs. C), 0.0001], CCL2 [Adj. P (LG vs. C), 0.004; Adj. P (HG vs. C), <0.0001], CCL4 [Adj. P (LG vs. C), 0.02; Adj. P (HG vs. C), 0.01], CCL5 [Adj. P (HG vs. C), 0.03] and PDGF-BB [Adj. P (HG vs. C), 0.01]. Furthermore, the progression of tumor from LG to HG was associated with significant increase in the levels of IL-6 and CXCL10 [Adj. P (LG vs. HG) =0.04 and =0.01, respectively] (Fig. 3).
Plasma cytokine profile of patients with ccRCC
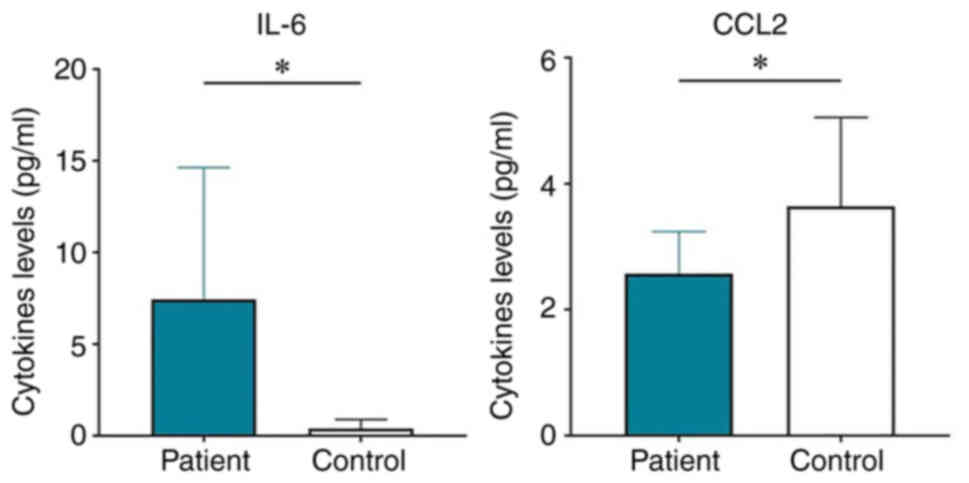
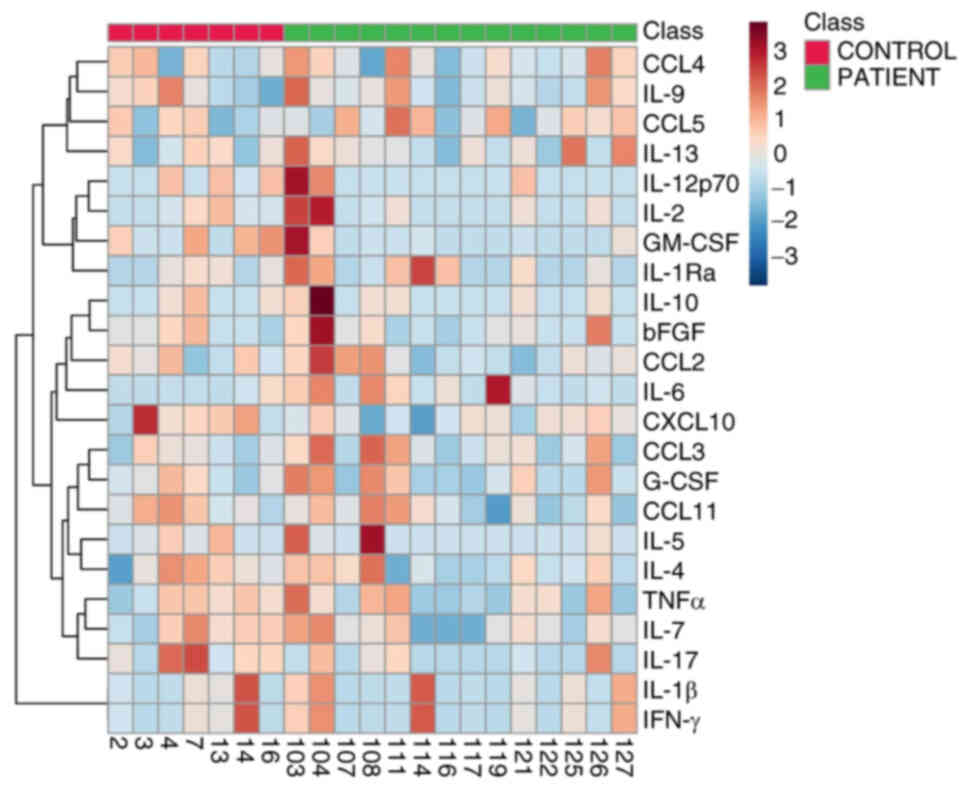
After comparing plasma samples from patients with ccRCC with healthy control plasma, significant differences were observed in the levels of IL-6 and CCL2. While IL-6 was higher in patients than in controls (P-value/R2=0.03/0.29), the opposite was shown for CCL2 (P/R2=0.04/0.23) (Fig. 4). Table SIV provides the size of statistical power of these significant findings. A graphical representation of the magnitude of the levels of all circulating cytokines in patients and controls is shown in a heatmap (Fig. 5). No significant correlation was observed between plasma and U0 samples (Table SV). In addition, a comparison between plasma cytokines from male and female patients was performed, but no significant effect of sex on plasma cytokine profile was detected (Table SVI)
Discussion
It is well known that inflammation is one of the major hallmarks of cancer (21), and an inflammatory microenvironment abundant in various signaling molecules, particularly cytokines, is a characteristic feature of RCC. It has been suggested that the inflammatory profile may be useful for predicting prognosis, survival and/or treatment response in patients with this type of tumor (22). Although there are some previous data on serum cytokines in RCC (15), the cytokine profile of urine from patients with ccRCC is not fully clear. Therefore, the present study aimed to investigate the role of urinary and circulating cytokines in the pathogenesis of ccRCC, as well as their potential capacity to be used for noninvasive diagnosis of ccRCC. Furthermore, the present study examined the effect of surgery and tumor grade on the urinary cytokine profile.
Regarding the potential diagnostic and predictive value of urinary cytokines in ccRCC, the present study found that IL-1Ra, IL-6, IL-15, CCL2, CCL3, CCL4, CXCL10, GM-CSF and PDGF-BB reached significantly higher levels in the urine of patients prior to operation compared with those in control urine. Since changes in urinary immune profile reflect ongoing pathological processes in the kidney (23–25), increased levels of these cytokines may be indicative of their tumorigenic character. On the other hand, the cytokine profile of urine may not always exactly match the immune changes occurring in ccRCC tissue, as demonstrated in the case of plasma cytokines (15). Therefore, several factors need to be considered when interpreting the current results. Firstly, cytokines have a short half-life and their resulting concentration measured in urine samples may be influenced by the presence of cytokine-binding proteins, inhibitors, soluble cytokine receptors and/or the method of sample processing (26). In addition, some cytokines, after binding to their target receptor in tissue, may be internalized and subsequently degraded (27), implying that the levels of urinary cytokines may represent only a fraction of their originally secreted levels. Finally, individual cytokines may have different kidney excretion rates (28). Even if the cytokine profiles of urine and tissue were correlated, another important fact that should be considered is that numerous cytokines do not have a clearly defined character in the TME and can have either tumor-promoting or tumor-suppressing effects, depending on the tissue context. Therefore, further investigation is needed to determine the effect of each cytokine on the development of ccRCC in order to uncover the underlying mechanism of each of them in the pathogenesis of ccRCC. Nevertheless, it could be hypothesized that IL-1Ra, IL-6, IL-15, CCL2, CCL3, CCL4, CXCL10, GM-CSF and PDGF-BB may be promising candidates for urinary markers of ccRCC.
Notably, presurgical urine, as well as urine from patients with HG cancer, contained significantly lower levels of G-CSF than urine from control subjects. It has been shown that the hematopoietic factor G-CSF can be secreted by tumor cells, and that it contributes to tumor aggression and is negatively associated with prognosis (29). The tumor-promoting character of G-CSF has also been proposed in the context of ccRCC. Patients with nonmetastatic ccRCC and high intratumoral expression levels of G-CSF have been reported to be more likely to have higher grade tumors and a higher probability of experiencing recurrence (30). However, it remains unclear why healthy subjects exhibited higher levels of G-CSF in urine than patients with ccRCC in the present study, and whether these values are outside the physiological range or not, since the cut-off values of individual cytokines have not yet been established (31).
To determine the possible predictive ability of the examined molecules, the current study further evaluated the changes in their levels regarding tumor grade. The analysis further revealed that both patients with LG and HG cancer had significantly higher levels of IL-1Ra, IL-6, IL-15, CCL2 and CCL4 in their urine compared with controls. However, the levels of these cytokines (except IL-6) did not change significantly with the increase of tumor grade. These findings suggest that these cytokines probably would not have much clinical value in terms of tumor stage prediction in patients with ccRCC. By contrast, the results of PDGF-BB, CCL5, CXCL10, GM-CSF and G-CSF with regard to tumor stage appeared to be more clinically relevant and thus may deserve more attention. These cytokines were detected in significantly different concentrations only in one group of patients, either LG (GM-CSF) or HG (the remaining cytokines), compared with those in the control group. This may indicate that they represent a promising novel tool for distinguishing an advanced form of ccRCC from a localized one, and subsequently may enable us to estimate the prognosis of patients. Additionally, CXCL10 reached a significantly higher concentration in HG urine than in LG urine, whereas IL-6 levels were higher in both stages compared with the controls and were also significantly increased with tumor dedifferentiation, which suggests that these two could be potential markers of tumor progression. Previous reports seem to support our assumption, as the majority of the aforementioned cytokines have been suggested to have tumorigenic effects. PDGF-BB, a major mitogenic factor, serves a crucial role in both physiological and pathological blood vessel development, including tumor angiogenesis (32). Aberrant PDGF signaling contributes to several cancer hallmarks and has been detected in several human tumors (33,34). Its tumor-promoting effects in RCC underscore the common use of various PDGF receptor inhibitors in the treatment of patients with an advanced stage of this disease (35). CCL5 is an inflammatory chemokine, and pathologically increased activity of the CCL5/C-C chemokine receptor type 5 axis is considered to facilitate tumor progression through various mechanisms (36). The pro-tumorigenic role of CCL5, and its association with tumor pathological stage and grade, have also been demonstrated in ccRCC tissue (37). CXCL10 signaling increases cancer cell proliferation and angiogenesis (38), and its high expression in RCC tissue and association with poor survival have also been demonstrated (39,40). IL-6 is a potent proinflammatory mediator that is involved in chronic inflammation and carcinogenesis, and is responsible for cancer progression and maintenance of immune reactions (41,42). IL-6 enhances tumor cell proliferation, promotes tumor survival and the formation of metastasis (43), and has been reported to act as an important regulator of RCC pathogenesis (44).
Regarding GM-CSF, the situation appears to be more complicated compared with that of the other investigated cytokines. Significantly higher levels of this hematopoietic factor were detected in LG urine compared with in control urine, but this trend disappeared in HG urine. This may indicate that GM-CSF, in contrast to the aforementioned cytokines, could be a potential marker of localized ccRCC, and that its role in the development of ccRCC may change during tumor progression. Notably, previous studies have demonstrated that GM-CSF has a dual role. The inhibitory effects of GM-CSF on tumor growth and metastasis have been described; however, there is also evidence to suggest that, in multiple types of cancer, it acts as an immune-independent tumor promoter (45,46). Nevertheless, the present results concerning the predictive role of urinary cytokines in ccRCC are only preliminary and further investigation is needed before any definitive conclusions can be drawn.
When comparing preoperative and postoperative urine samples with control samples, the present study revealed that the urinary cytokine profile of patients changed only minimally after tumor removal. IL-1Ra, IL-6, IL-15, CCL2, CCL3, CCL4, CXCL10, GM-CSF and PDGF-BB levels were significantly higher in urine both before and after surgery compared with those in control urine. This may imply that a longer time is needed to detect any significant changes in the urinary cytokine profile of patients with ccRCC after tumor removal. Furthermore, it may be assumed that these cytokines are not only involved in the pathogenesis of ccRCC, but also in the local immune defensive and reparative processes occurring in the kidney tissue after surgical injury. For example, PDGF is known to serve a major role in almost all stages of wound healing (47) and the majority of the other aforementioned cytokines have been reported to participate in the acute phase of the inflammatory response, which peaks between the second and third day after injury (48).
There were three cytokines that reached markedly different levels in only one type of urine sample with respect to the controls, namely IL-5, VEGF and G-CSF. As the urinary levels of IL-5 and VEGF were higher on the 3rd day after surgery, it may be hypothesized that these molecules could be involved in the inflammatory and wound-healing processes occurring in the kidney after tumor resection, rather than in the pathogenesis of ccRCC. This notion seems plausible considering that IL-5 is the primary regulator of differentiation, maturation, expansion, survival and activation of eosinophils (49), which have critical roles in tissue healing and can promote angiogenesis (50,51). Thus, an increase in IL-5 shortly after surgery may be associated with the recruitment of eosinophils to the site of injury, where they participate in various reparatory processes. Regarding VEGF, this factor is considered to be the most important regulator of angiogenesis (52). Compared with other angiogenic growth factors, such as bFGF and TGF-β, VEGF has been reported to affect not only the formation of blood vessels, but also other important processes of the wound-healing cascade, such as epithelization and collagen deposition (53). The maximal VEGF activity in the wound is estimated to occur between days 3 and 7 after injury (54). As for G-CSF, its levels were lower in urine samples from patients before surgery compared with those in samples from the controls, but this trend disappeared after removal of the tumor. Based on this, it can be assumed that this hematopoietic factor may be involved in the antitumor immunity of the host. The decreased levels of G-CSF in presurgical urine samples could possibly reflect tumor-induced suppression of the immune system of the host, which, after tumor removal, was restored. However, this does not appear to be in line with its character proposed by previous studies (29,30) as aforementioned.
Regarding the diagnostic value of plasma cytokines in ccRCC, the results of the present study showed that the plasma and urine cytokine profiles of patients with ccRCC were markedly different. While in urine changes in the levels of 10 cytokines were observed, in plasma only two cytokines exhibited significant changes. This may be explained by the fact that two different control groups were used as a reference for urine and plasma analysis. Additionally, when correlation analysis was performed, no statistically significant correlation was detected between plasma and presurgical urine samples from patients. However, due to the low number of samples in the present dataset, only 11 matched plasma-urine samples could be investigated and, since the detection rate of cytokines in patient plasma was relatively low, the test could not be performed on all of the examined molecules. There are limited reports on the immune profiles of plasma and urine in ccRCC. However, Nobles et al (55), who studied a population with low levels of inflammation, did not find any correlation or association between plasma and urinary cytokine profiles. Thus, further research is warranted to accurately determine whether in patients with ccRCC the changes in the immune profile of plasma are somehow related to the changes in urine.
Regarding the potential plasma markers of ccRCC, the present study detected significantly higher levels of IL-6 in patients compared with those in the controls. This is in line with the results of previous reports. An increase in the systemic levels of IL-6 has also been observed in other patients with different types of cancer, and this feature has been revealed to be positively associated with tumor stage and negatively associated with prognosis (56). High levels of IL-6 in serum and its prognostic value have also been reported in patients with RCC (16,57–60). The current study observed lower levels of CCL2 in patient plasma than in the controls. This observation is notable, since CCL2 is mostly considered to be a tumor-promoting chemokine since it serves a critical role in the progression of various cancer types by supporting several major tumor-promoting processes (61,62). High levels of CCL2 expression and its correlation with advanced stage and recurrence have also been reported in ccRCC (63–65). Additionally, circulating CCL2 has been suggested as a marker of breast (66) and prostate (67) cancer. On the other hand, there is evidence that CCL2 is able to enhance the antitumor capability of monocytes and neutrophils (68), which could explain the present findings. It may be hypothesized that, in the case of ccRCC, CCL2 could serve a role in the immune defensive mechanisms of the host, which are suppressed by the tumor.
With regard to the analyses presented in Tables SII and SIII, the sample size used was relatively low. Therefore, none of these results were considered to be statistically and/or clinically relevant. Further research is needed to elucidate more deeply the dynamics of urinary cytokines in patients with ccRCC after surgery, particularly to determine whether the trend observed in CCL2 is also preserved even if the number of samples increases, and to identify potential markers of recurrence.
The present study has several limitations, including: i) The number of participants, and the control and patients' datasets were limited, and it would be advisable to verify the present results using a larger dataset; ii) due to a low number of samples from patients with different tumor grades, it was not possible to perform more detailed analysis of cytokine levels with respect to this clinical feature; iii) for monitoring the urinary cytokine dynamics after surgery, the time interval of sample collection appears to be insufficient and it would be advisable to increase it; iv) the control group for urine analysis consisted of different subjects compared with the control group for plasma analysis, which may have affected the present observations; and v) monitoring plasma cytokines after surgery would further increase the quality of the current study.
In conclusion, the results of the present study suggested that G-CSF, IL-1Ra, IL-6, IL-15, CCL2, CCL3, CCL4, CXCL10, GM-CSF and PDGF-BB may represent promising non-invasive biomarkers of ccRCC. G-CSF, GM-CSF, IL-6, CXCL10, CCL5 and PDGF-BB could potentially be used to determine the grade of ccRCC and consequently estimate the prognosis of patients with this cancer type. Furthermore, the levels of circulating CCL2 and IL-6 may have potential diagnostic value for ccRCC. The decrease in the urinary G-CSF and circulating CCL2 levels in patients with this tumor type requires further research.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Ms. Katarína Jesenská from the Department of Pharmacology, Jessenius Faculty of Medicine in Martin, for technical support.
Funding
The present study was funded by the Scientific Grant Agency of the Ministry of Education, Research, Development and Youth of the Slovak Republic and the Slovak Academy of Sciences (grant nos. VEGA 1/0428/21, VEGA 1/0072/23 and APVV-19-0033), and the integrated operational program infrastructure for the project New Possibilities for the Management of Serious Diseases in Medical and Preventive Care with Regard to the Safety of Health Professionals (grant no. ITMS: 313011AUA5), which was co-financed by the European Regional Development Fund.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
DV and MŠ conceptualized the study. DV, MD, JĽ, JD, LB, IŠ, SF and PS designed the methodology of the study. DV analyzed the data using statistical software. DV and MD verified the experimental data and results. JD, LB, JĽ, JŠ and PS provided the biological materials. DV and MŠ interpreted the data. DV wrote the original draft manuscript, and MD, MŠ and SF reviewed, edited the manuscript and gave a final approval of the version to be published. DV prepared the visual representation of the data. MŠ supervised the whole study, while DV managed and coordinated the planning of the research activity and its execution. MŠ, SF and JŠ were responsible for acquisition of the financial support for the project. DV and MŠ confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and informed consent to participate
The present study was carried out in accordance with The Declaration of Helsinki and was approved by the Institutional Ethics Committee of the Jessenius Faculty of Medicine of Martin Comenius University in Bratislava (approval no. 19/2019). The collection and handling of biological material were conducted in accordance with Slovakian and European legislation. Each participant in the study provided written informed consent prior to the start of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Ricketts CJ, De Cubas AA, Fan H, Smith CC, Lang M, Reznik E, Bowlby R, Gibb EA, Akbani R, Beroukhim R, et al: The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep. 23:36982018. View Article : Google Scholar : PubMed/NCBI | |
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 71:209–249. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O and Bray F: Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 103:356–387. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Padala SA and Barsouk A, Thandra KC, Saginala K, Mohammed A, Vakiti A, Rawla P and Barsouk A: Epidemiology of renal cell carcinoma. World J Oncol. 11:79–87. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Vasudev NS, Wilson M, Stewart GD, Adeyoju A, Cartledge J, Kimuli M, Datta S, Hanbury D, Hrouda D, Oades G, et al: Challenges of early renal cancer detection: Symptom patterns and incidental diagnosis rate in a multicentre prospective UK cohort of patients presenting with suspected renal cancer. BMJ Open. 10:e0359382020. View Article : Google Scholar : PubMed/NCBI | |
|
Bradley AJ, Maskell GF, Mannava A, Pollard A and Welsh T: Routes to diagnosis and missed opportunities in the detection of renal cancer. Clin Radiol. 76:129–134. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Yin Q, Xu H, Zhong Y, Ni J and Hu S: Diagnostic performance of MRI, SPECT, and PET in detecting renal cell carcinoma: A systematic review and meta-analysis. BMC Cancer. 22:1632022. View Article : Google Scholar : PubMed/NCBI | |
|
Usher-Smith J, Simmons RK, Rossi SH and Stewart GD: Current evidence on screening for renal cancer. Nat Rev Urol. 17:637–642. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Rossi SH, Blick C, Handforth C, Brown JE and Stewart GD: Essential research priorities in renal cancer: A modified delphi consensus statement. Eur Urol Focus. 6:991–998. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang S, Zhang E, Long J, Hu Z, Peng J, Liu L, Tang F, Li L, Ouyang Y and Zeng Z: Immune infiltration in renal cell carcinoma. Cancer Sci. 110:1564–1572. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Díaz-Montero CM, Rini BI and Finke JH: The immunology of renal cell carcinoma. Nat Rev Nephrol. 16:721–735. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Kartikasari AER, Huertas CS, Mitchell A and Plebanski M: Tumor-Induced inflammatory cytokines and the emerging diagnostic devices for cancer detection and prognosis. Front Oncol. 11:6921422021. View Article : Google Scholar : PubMed/NCBI | |
|
Lan T, Chen L and Wei X: Inflammatory cytokines in cancer: Comprehensive Understanding and Clinical Progress in Gene Therapy. Cells. 10:1002021. View Article : Google Scholar : PubMed/NCBI | |
|
Zeng Q, Sun S, Li Y, Li X, Li Z and Liang H: Identification of therapeutic targets and prognostic biomarkers among CXC chemokines in the renal cell carcinoma microenvironment. Front Oncol. 9:15552020. View Article : Google Scholar : PubMed/NCBI | |
|
Lee MH, Laajala E, Kreutzman A, Järvinen P, Nísen H, Mirtti T, Hollmén M and Mustjoki S: The tumor and plasma cytokine profiles of renal cell carcinoma patients. Sci Rep. 12:134162022. View Article : Google Scholar : PubMed/NCBI | |
|
Gudbrandsdottir G, Aarstad HH, Bostad L, Hjelle KM, Aarstad HJ, Bruserud Ø, Tvedt THA and Beisland C: Serum levels of the IL-6 family of cytokines predict prognosis in renal cell carcinoma (RCC). Cancer Immunol Immunother. 70:19–30. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Vargová D, Dargaj J, Fraňová S, Dohál M, Ľupták J, Švihra J, Briš L, Grendár M and Šutovská M: Immunobiochemical profile of clear cell renal cell carcinoma (ccRCC): A preliminary study. Gen Physiol Biophys. 42:387–401. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and Winchester DP: The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more ‘personalized’ approach to cancer staging. CA Cancer J Clin. 67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE and Ulbright TM: The 2016 WHO classification of tumours of the urinary system and male genital Organs-Part A: Renal, penile, and testicular tumours. Eur Urol. 70:93–105. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Faul F, Erdfelder E, Buchner A and Lang AG: Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 41:1149–1160. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Colotta F, Allavena P, Sica A, Garlanda C and Mantovani A: Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 30:1073–1081. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
de Vivar Chevez AR, Finke J and Bukowski R: The Role of Inflammation in Kidney Cancer. Adv Exp Med Biol. 816:197–234. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Gradin A, Andersson H, Luther T, Anderberg SB, Rubertsson S, Lipcsey M, Åberg M, Larsson A, Frithiof R and Hultström M: Urinary cytokines correlate with acute kidney injury in critically ill COVID-19 patients. Cytokine. 146:1555892021. View Article : Google Scholar : PubMed/NCBI | |
|
Martinez Valenzuela L, Draibe J, Bestard O, Fulladosa X, Gómez-Preciado F, Antón P, Nadal E, Jové M, Cruzado JM and Torras J: Urinary cytokines reflect renal inflammation in acute tubulointerstitial nephritis: A multiplex bead-based assay assessment. J Clin Med. 10:29862021. View Article : Google Scholar : PubMed/NCBI | |
|
Jakiela B, Kosałka J, Plutecka H, Węgrzyn AS, Bazan-Socha S, Sanak M and Musiał J: Urinary cytokines and mRNA expression as biomarkers of disease activity in lupus nephritis. Lupus. 27:1259–1270. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Liu C, Chu D, Kalantar-Zadeh K, George J, Young HA and Liu G: Cytokines: From clinical significance to quantification. Adv Sci (Weinh). 8:e20044332021. View Article : Google Scholar : PubMed/NCBI | |
|
Cendrowski J, Mamińska A and Miaczynska M: Endocytic regulation of cytokine receptor signaling. Cytokine Growth Factor Rev. 32:63–73. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Sugama K, Suzuki K, Yoshitani K, Shiraishi K and Kometani T: Urinary excretion of cytokines versus their plasma levels after endurance exercise. Exerc Immunol Rev. 19:29–48. 2013.PubMed/NCBI | |
|
Karagiannidis I, Salataj E, Said Abu Egal E and Beswick EJ: G-CSF in tumors: Aggressiveness, tumor microenvironment and immune cell regulation. Cytokine. 142:1554792021. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Z, Zhu Y, Wang Y, Fu Q, Fu H, Wang Z, Zhang J, Li G, Xu J and Dai B: Prognostic value of granulocyte colony-stimulating factor in patients with non-metastatic clear cell renal cell carcinoma. Oncotarget. 8:69961–69971. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Monastero RN and Pentyala S: Cytokines as biomarkers and their respective clinical cutoff levels. Int J Inflam. 2017:43094852017.PubMed/NCBI | |
|
Raica M and Cimpean AM: Platelet-Derived growth factor (PDGF)/PDGF receptors (PDGFR) Axis as target for antitumor and antiangiogenic therapy. Pharmaceuticals. 3:572–599. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Liu KW, Hu B and Cheng SY: Platelet-derived growth factor signaling in human malignancies. Chin J Cancer. 30:581–584. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Bartoschek M and Pietras K: PDGF family function and prognostic value in tumor biology. Biochem Biophys Res Commun. 503:984–990. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Powles T, Albiges L, Bex A, Grünwald V, Porta C, Procopio G, Schmidinger M, Suárez C and de Velasco G; ESMO Guidelines Committee. Electronic address, : simpleclinicalguidelines@esmo.org: ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Ann Oncol. 32:1511–1519. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Aldinucci D, Borghese C and Casagrande N: The CCL5/CCR5 Axis in cancer progression. Cancers (Basel). 12:17652020. View Article : Google Scholar : PubMed/NCBI | |
|
Bai S, Wu Y, Yan Y, Kang H, Zhang J, Ma W, Gao Y, Hui B, Li R, Zhang X and Ren J: The effect of CCL5 on the immune cells infiltration and the prognosis of patients with kidney renal clear cell carcinoma. Int J Med Sci. 17:2917–2925. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Tokunaga R, Zhang W, Naseem M, Puccini A, Berger MD, Soni S, McSkane M, Baba H and Lenz HJ: CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation-A target for novel cancer therapy. Cancer Treat Rev. 63:40–47. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Wightman SC, Uppal A, Pitroda SP, Ganai S, Burnette B, Stack M, Oshima G, Khan S, Huang X, Posner MC, et al: Oncogenic CXCL10 signalling drives metastasis development and poor clinical outcome. Br J Cancer. 113:327–335. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Qu G, Wang H, Yan H, Liu G and Wu M: Identification of CXCL10 as a prognostic biomarker for clear cell renal cell carcinoma. Front Oncol. 12:8576192022. View Article : Google Scholar : PubMed/NCBI | |
|
Hunter CA and Jones SA: IL-6 as a keystone cytokine in health and disease. Nat Immunol. 16:448–457. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Schumacher N, Schmidt S, Schwarz J, Dohr D, Lokau J, Scheller J, Garbers C, Chalaris A, Rose-John S and Rabe B: Circulating soluble IL-6R but Not ADAM17 activation drives mononuclear cell migration in tissue inflammation. J Immunol. 197:3705–3715. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Rašková M, Lacina L, Kejík Z, Venhauerová A, Skaličková M, Kolář M, Jakubek M, Rosel D, Smetana K Jr and Brábek J: The role of IL-6 in cancer cell invasiveness and metastasis-overview and therapeutic opportunities. Cells. 11:36982022. View Article : Google Scholar : PubMed/NCBI | |
|
Kamińska K, Czarnecka AM, Escudier B, Lian F and Szczylik C: Interleukin-6 as an emerging regulator of renal cell cancer. Urol Oncol. 33:476–485. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Hong IS: Stimulatory versus suppressive effects of GM-CSF on tumor progression in multiple cancer types. Exp Mol Med. 48:e242. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Kumar A, Taghi Khani A, Sanchez Ortiz A and Swaminathan S: GM-CSF: A Double-Edged sword in cancer Immunotherapy. Front Immunol. 13:9012772022. View Article : Google Scholar : PubMed/NCBI | |
|
Werner S and Grose R: Regulation of wound healing by growth factors and cytokines. Physiol Rev. 83:835–870. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Strang H, Kaul A, Parikh U, Masri L, Saravanan S, Li H, Miao Q and Balaji S: Role of cytokines and chemokines in wound healing. Wound Healing, Tissue Repair, and Regeneration in Diabetes. Elsevier Academic Press; Cambridge, MA: pp. 197–235. 2020, View Article : Google Scholar | |
|
Ray A: Cytokines and their Role in Health and Disease: A Brief Overview. MOJ Immunol. 42016. | |
|
Kim HJ and Jung Y: The emerging role of eosinophils as multifunctional leukocytes in health and disease. Immune Netw. 20:e242020. View Article : Google Scholar : PubMed/NCBI | |
|
Coden ME and Berdnikovs S: Eosinophils in wound healing and epithelial remodeling: Is coagulation a missing link? J Leukoc Biol. 108:93–103. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Holmes DI and Zachary I: The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 6:2092005. View Article : Google Scholar : PubMed/NCBI | |
|
Stojadinovic O, Kodra A, Golinko M, Tomic-Canic M and Brem H: A Novel, Non-Angiogenic, Mechanism of Vegf: Stimulation of Keratinocyte and Fibroblast Migration. Wound Repair Regen. 15:A302007.PubMed/NCBI | |
|
Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP and Brem H: The role of vascular endothelial growth factor in wound healing. J Surg Res. 153:347–358. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Nobles C, Bertone-Johnson ER, Ronnenberg AG, Faraj JM, Zagarins S, Takashima-Uebelhoer BB and Whitcomb BW: Correlation of urine and plasma cytokine levels among reproductive-aged women. Eur J Clin Invest. 45:460–465. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Lippitz BE and Harris RA: Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. Oncoimmunology. 5:e10937222016. View Article : Google Scholar : PubMed/NCBI | |
|
Blay JY, Negrier S, Combaret V, Attali S, Goillot E, Merrouche Y, Mercatello A, Ravault A, Tourani JM, Moskovtchenko JF, et al: Serum level of interleukin 6 as a prognosis factor in metastatic renal cell carcinoma. Cancer Res. 52:3317–3322. 1992.PubMed/NCBI | |
|
Cechim G, Ellwanger JH, Kaminski V de L, Berger M and Chies JAB: Increased systemic IL-6 levels point to inflammation as a determinant of renal cell carcinoma development. Clin Biomed Res. 41:1–211. 2021. | |
|
Negrier S, Perol D, Menetrier-Caux C, Escudier B, Pallardy M, Ravaud A, Douillard JY, Chevreau C, Lasset C and Blay JY; Groupe Francais d'Immunotherapie, : Interleukin-6, Interleukin-10, and Vascular Endothelial Growth Factor in Metastatic Renal Cell Carcinoma: Prognostic Value of Interleukin-6-From the Groupe Français d'Immunothérapie. J Clin Oncol. 22:2371–2378. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y and Zhang Y: Prognostic role of interleukin-6 in renal cell carcinoma: A meta-analysis. Clin Transl Oncol. 22:835–843. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Xu M, Wang Y, Xia R, Wei Y and Wei X: Role of the CCL2-CCR2 signalling axis in cancer: Mechanisms and therapeutic targeting. Cell Prolif. 54:e131152021. View Article : Google Scholar : PubMed/NCBI | |
|
Korbecki J, Grochans S, Gutowska I, Barczak K and Baranowska-Bosiacka I: CC Chemokines in a Tumor: A review of pro-cancer and anti-cancer properties of receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 ligands. Int J Mol Sci. 21:76192020. View Article : Google Scholar : PubMed/NCBI | |
|
Arakaki R, Yamasaki T, Kanno T, Shibasaki N, Sakamoto H, Utsunomiya N, Sumiyoshi T, Shibuya S, Tsuruyama T, Nakamura E, et al: CCL2 as a potential therapeutic target for clear cell renal cell carcinoma. Cancer Med. 5:2920–2933. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z, Xie H, Zhou L, Liu Z, Fu H, Zhu Y, Xu L and Xu J: CCL2/CCR2 axis is associated with postoperative survival and recurrence of patients with non-metastatic clear-cell renal cell carcinoma. Oncotarget. 7:51525–51534. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Y, Zhai C, Chang Y, Zhou L, Shi T, Tan C, Xu L and Xu J: High expression of chemokine CCL2 is associated with recurrence after surgery in clear-cell renal cell carcinoma. Urol Oncol. 34:238.e19–e26. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Lubowicka E, Przylipiak A, Zajkowska M, Piskór BM, Malinowski P, Fiedorowicz W and Ławicki S: Plasma chemokine CCL2 and its receptor CCR2 concentrations as diagnostic biomarkers for breast cancer patients. Biomed Res Int. 2018:21243902018. View Article : Google Scholar : PubMed/NCBI | |
|
Izumi K, Mizokami A, Lin HP, Ho HM, Iwamoto H, Maolake A, Natsagdorj A, Kitagawa Y, Kadono Y, Miyamoto H, et al: Serum chemokine (CC motif) ligand 2 level as a diagnostic, predictive, and prognostic biomarker for prostate cancer. Oncotarget. 7:8389–8398. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Jin J, Lin J, Xu A, Lou J, Qian C, Li X, Wang Y, Yu W and Tao H: CCL2: An important mediator between tumor cells and host cells in tumor microenvironment. Front Oncol. 11:7229162021. View Article : Google Scholar : PubMed/NCBI |