Personalized circulating tumor DNA response to local radiotherapy in a patient with an early lobular breast cancer: A case report
- Authors:
- Published online on: April 25, 2024 https://doi.org/10.3892/ol.2024.14415
- Article Number: 282
Abstract
Introduction
Breast cancer (BC) is the most prevalent type of cancer and the second highest cause of cancer mortality in women worldwide with an estimated 2.3 million new cases and >685,000 deaths reported in 2020 (1). Invasive lobular carcinoma (ILC) is the second most common histologic subtype of breast cancer worldwide, accounting for 10–15% of all breast cancer cases (2,3). Over the past two decades, the incidence of ILC has increased, likely due to improvements in diagnostic techniques in breast imaging (4). ILC is a distinct entity with unique clinical and molecular features, including a diffuse, multifocal, and poorly circumscribed growth pattern, and is associated with difficult and delayed early diagnosis, inherent resistance to conventional therapies and a risk of late recurrence, which all present significant challenges in management (5). As with other breast cancers, the treatment of early lobular BC involves conservative surgery or ‘lumpectomy’ followed by adjuvant radiation. However, due to the multifocality and diffuse growth pattern, the rate of positive margins and thus second revision surgeries is higher in invasive lobular carcinomas than in ductal carcinomas (6).
The purpose of radiation therapy in the adjuvant setting is to sterilize subclinical localized residual disease that persists after surgical resection but is undetectable by clinical or radiographic assays. Whole breast irradiation decreases the risk of ipsilateral breast cancer recurrence by ~50% (7–10) in both invasive ductal carcinoma and ILC. Moreover, partial breast irradiation of ILC is associated with higher ipsilateral breast cancer recurrence rates and is relatively contraindicated in ILC (11). Nevertheless, the majority of women with ILC treated with breast conserving surgery do not develop in-breast local recurrence, which suggests that the use of whole breast irradiation is overtreating these women. There is a clear clinical need for more effective biomarkers to predict the benefits of radiation for patients with cancer, including women with breast cancer.
The measurement of circulating tumor DNA (ctDNA) has emerged as a biomarker with wide-ranging applications in cancer management (12). ctDNA levels have both prognostic and predictive value in many cancers including breast cancer. Moreover, dynamic changes in ctDNA levels, including ctDNA clearance have been reported to be associated with response to various systemic therapies, such as PI3K inhibitors. The power of ctDNA detection derives from its role in the signaling of clinically occult metastatic disease or minimal residual disease (MRD). There are many approaches to measure ctDNA in the plasma, with the most sensitive approaches involving the sequencing of tumors followed by the development of tumor-specific assays personalized to the patient's tumor. These ‘tumor-bespoke’ assays use either sequencing or PCR reactions to identify the individual mutations in a patient's tumor (13,14). The Signatera™ assay uses tumor whole-exome sequencing to identify 16 patient-specific somatic single-nucleotide variants and short indels to generate multiplex PCR primer pairs. These pairs are used to amplify universal libraries, which are then sequenced on a NGS sequencing platform (HiSeq 2500 system; Illumina, Inc.) (15). The present study reports a case of invasive lobular carcinoma, in which ctDNA clearance suggested response to local breast radiation therapy.
Case report
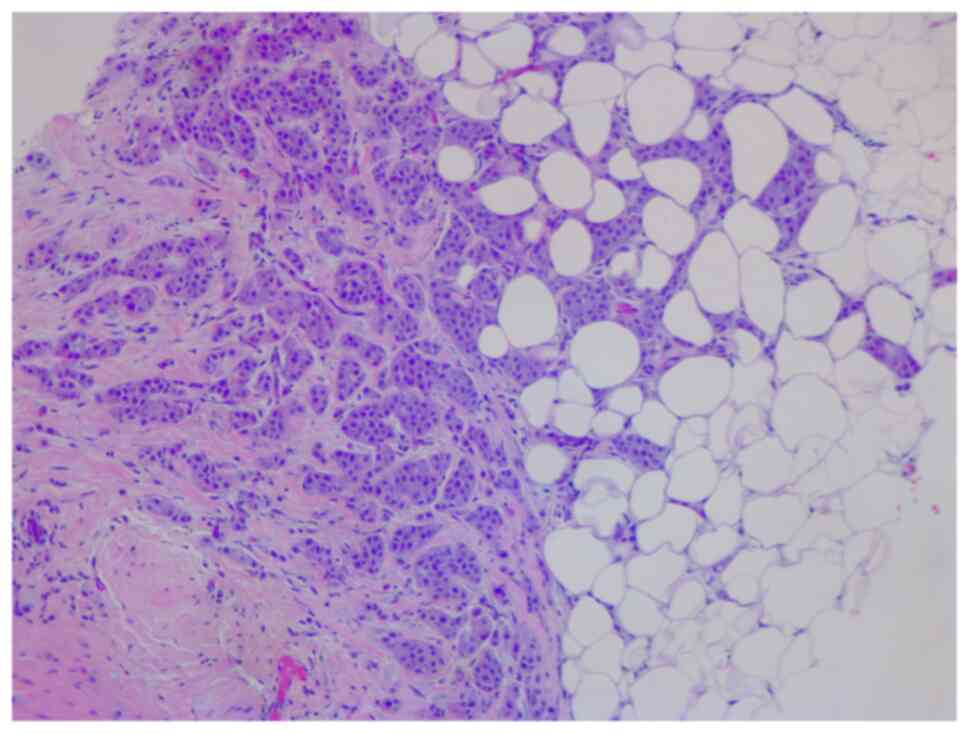
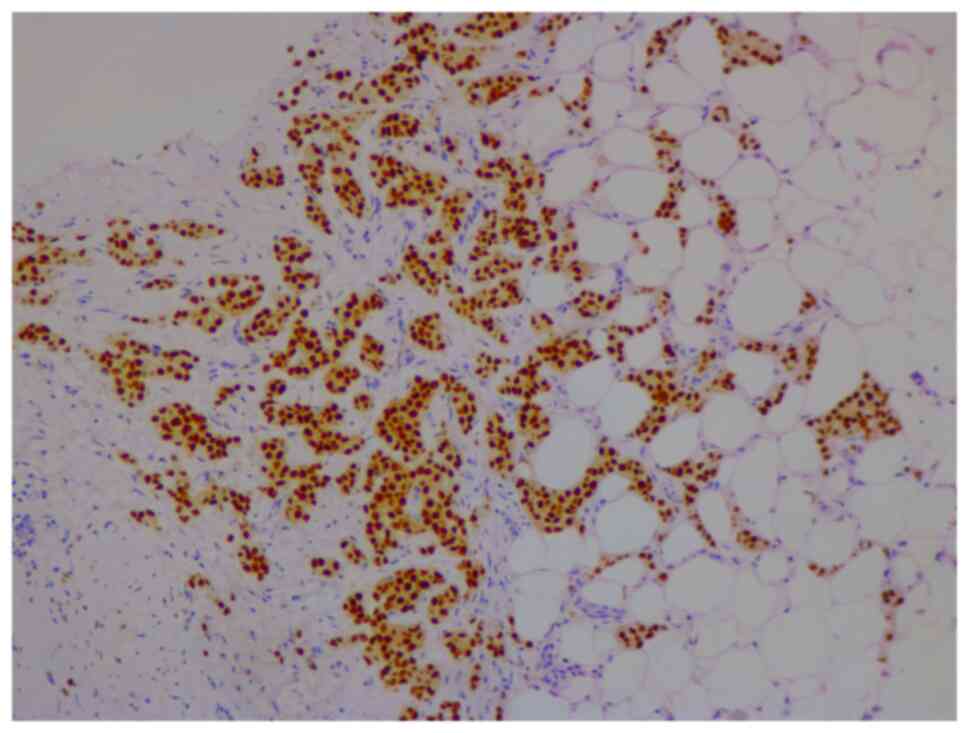
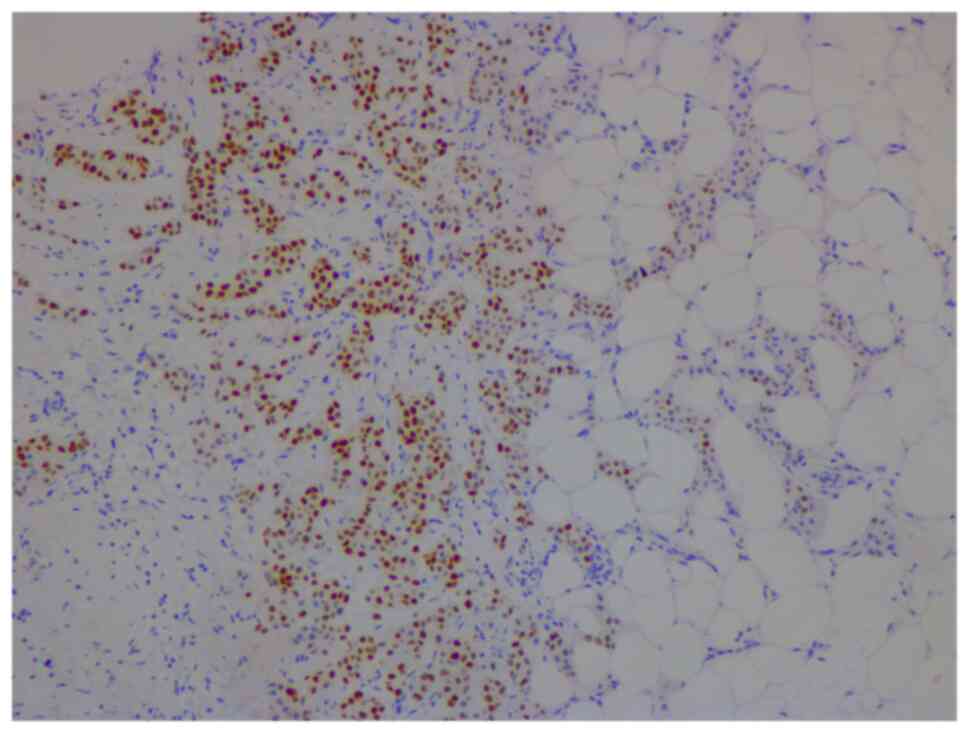
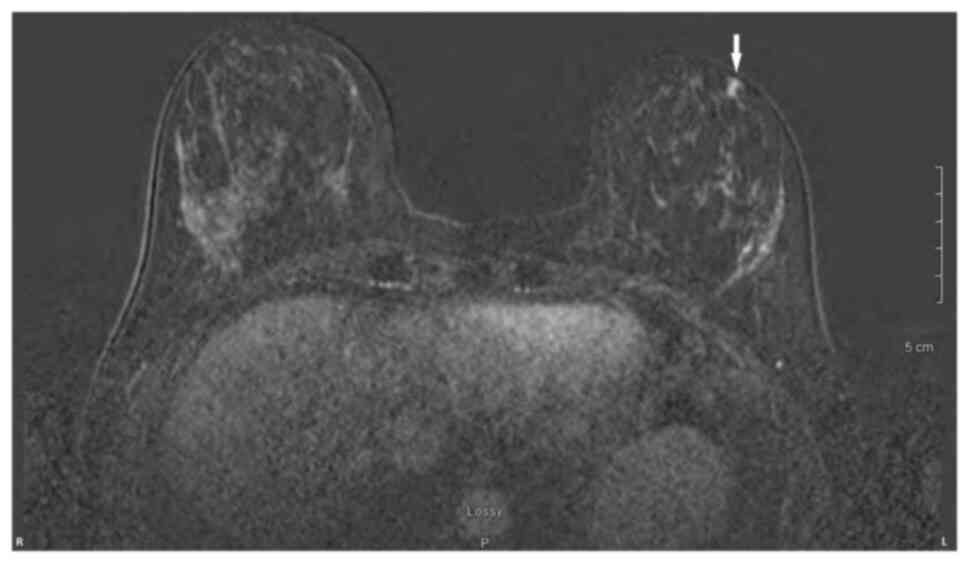
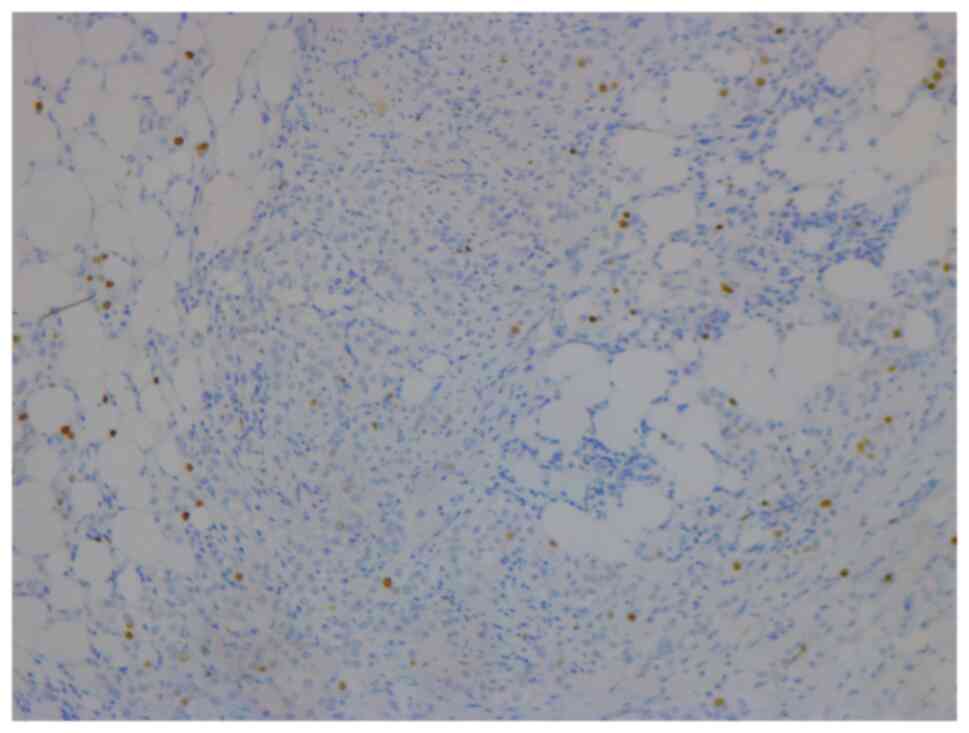
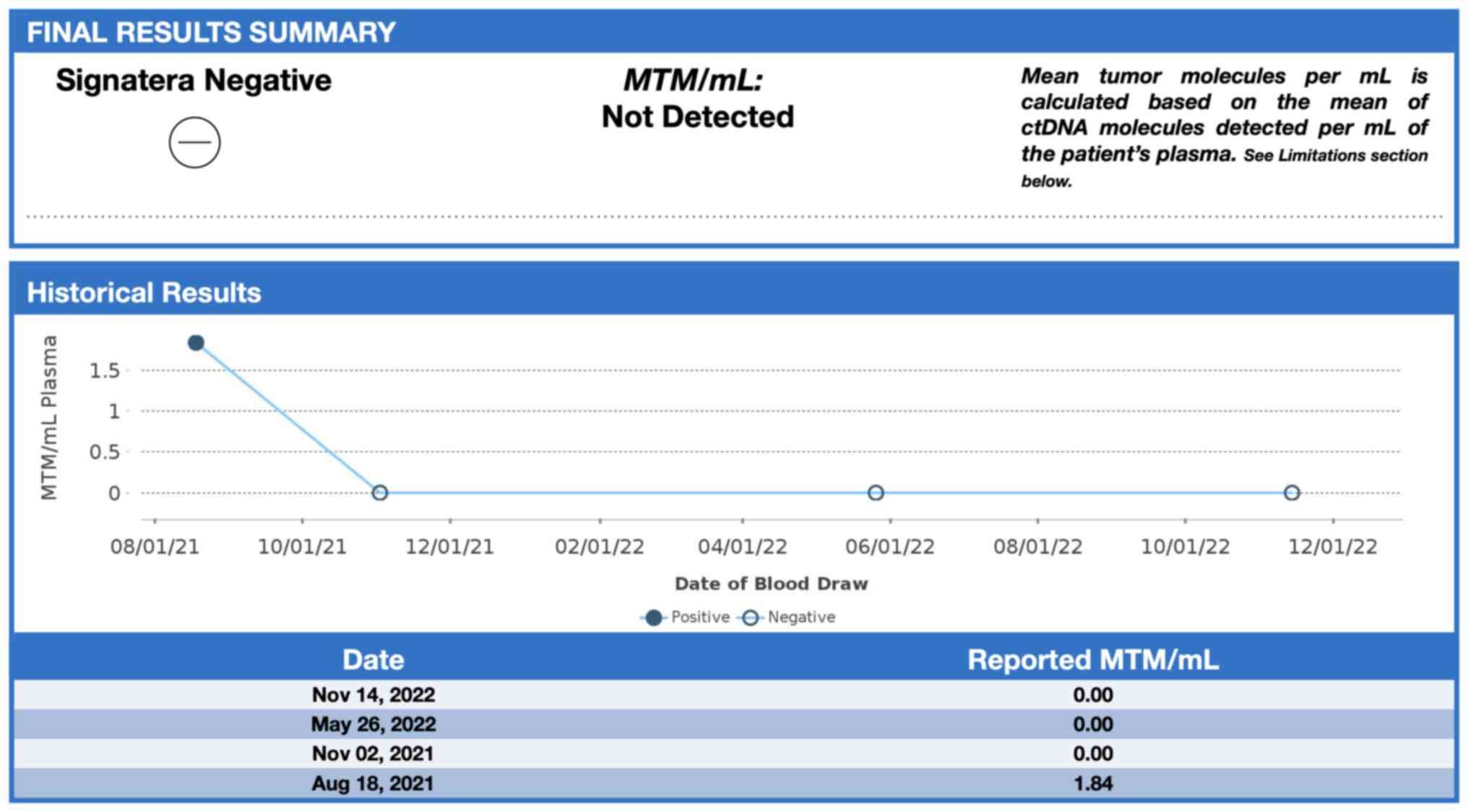
In January 2021, a 46-year-old pre-menopausal female, with a family history of breast cancer, presented with a palpable 1 cm (length) mass in her left breast with no palpable lymphadenopathy. The breast biopsy showed an invasive lobular carcinoma breast cancer (Fig. 1), Nottingham grade 2/3, estrogen (98%; Fig. 2) and progesterone (45%) receptor positive (Fig. 3), Her2 score 2+ based on the immunohistochemistry (according to ASCO-CAP clinical laboratory guidelines) (16), fluorescence in-situ hybridisation not amplified. Pre-operative breast MRI did not reveal any other suspicious focus of enhancement in the left breast but did reveal a 4×3×4 mm non-mass enhancement in the right breast, classified as a BI-RADS 4B lesion (17). A breast Magnetic resonance imaging (MRI) guided biopsy was done, which showed a lobular carcinoma in situ, Nottingham grade 2 (Fig. 4). In April 2021, the patient underwent 2 partial mastectomies, one on each breast, localized with radioactive seeds and sentinel lymph node biopsy in the left axilla. Pathological examination of the resection sample showed a 1 cm (length) grade 2 invasive lobular carcinoma, without lymphovascular invasion, with lobular carcinoma in-situ, negative margins and 0/4 sentinel lymph nodes on the left side (pT1pN0; AJCC-Breast Cancer Staging; 8th edition) (18). The right breast sample showed only lobular carcinoma in-situ with negative margins. The Ki67 staining of the invasive lobular carcinoma was 7–8% (Fig. 5), but the Oncotype DX® (Exact Sciences, Inc.) Breast Recurrence Score was 24. A germline test was performed on the blood samples of patients, which included DNA extraction, PCR amplification, sequencing, bioinformatics analysis and variant interpretation. The test revealed no clinically relevant pathogenic sequence variants in the BRCA1, BRCA2 and PALB2 genes. After tumor board discussion, based on the Oncotype Dx result, the hormonal status and age of the patient, adjuvant chemotherapy was offered but the patient refused. Concurrently, a personalized, tumor-informed multiplex-PCR (m-PCR)-NGS assay (Signatera™) was performed. The first sample was collected in August 2021 and the result was reported as a positive test (1.85 MTM/ml) (Fig. 6). Adjuvant therapy was again offered but the patient refused both chemotherapy and endocrine therapy (Tamoxifen) +/- ovarian suppression. Over 28 days in September and October 2021, the patient received adjuvant radiation therapy: A total of 42.4 Gy in 16 fractions was delivered to the whole left breast, followed by a boost to the surgical bed of 10 Gy in 4 fractions. After radiation therapy, 3 ctDNA analyses were performed in November 2021 and, May and November 2022 and all were reported as undetectable (0.00 MTM/ml) (Fig. 6). Tumor markers (CA15-3 and CA-125) were assessed every 6 months and were always negative. Computed tomography of the head was performed in November 2021 due to new symptoms (postural dizziness) and was negative for metastatic disease. The patient was followed up by the breast surgeon every 6 months, with her last visit was in November 2022 during which the physical exam was negative for local recurrence. The patient's last breast imaging was performed in March 2023 and both ultrasound and mammogram showed BI-RADS2 with benign findings. At the time of this report, the patient remains with no signs of local or distant recurrence of disease and excellent performance status and.
Clinical testing
In order to predict an early relapse after adjuvant treatment, the patient consented to periodic ctDNA monitoring in her blood. She was first tested 3 months after surgery and 1, 7 and 13 months after radiation therapy using the Signatera™ test, in addition to a physical exam every six months and a mammogram once a year.
Discussion
Breast cancer is a heterogeneous disease and ctDNA can accurately reflect this heterogeneity, supporting the detection, monitoring and understanding of the evolution of the disease (19). Previous studies have reported the value of ctDNA in detecting MRD, monitoring treatment response or resistance and predicting early relapse (20,21). Nevertheless, studies investigating the clinical utility of serial ctDNA monitoring for treatment guidance are few (22,23). It is important to note that the sensitivity and specificity of these assays varies depending on the method used to measure ctDNA in plasma. Many ctDNA assays use pre-defined recurrently mutated gene panels, which, for the purpose of tracking MRD have the limitation of missing mutations or alterations not included in or tested by the panel. Tumour ‘bespoke’ assays use the primary tumour to screen and select specific mutation variants belonging to the tumour. These highly sensitive and specific personalized assays are best used to detect and track MRD (24). These tumor bespoke assays provide accurate prognostic information related to distant disease recurrence, as evidence of micrometastatic disease. However, they appear to be less accurate in predicting local response to therapy (25). This is apparent in studies of ctDNA in the context of neoadjuvant treatment for breast cancer. For instance, in the large I-SPY study reported that early clearance of ctDNA was associated with pathological complete response (pCR) in the breast only in patients with triple negative breast cancer (26). Cavallone et al (27) reported that early clearance of ctDNA had a relatively low predictive value of 50% for pCR. These same assays are highly predictive of distant relapse free survival. In previous studies, all recurrences reported in patients with positive tumor bespoke assays were distant recurrences (21,28,29).
In the present study, the detection and quantification of breast cancer-derived ctDNA using a personalized tumor bespoke assay is reported. In this patient, with a small invasive lobular carcinoma in a background of lobular carcinoma in situ (LCIS), the commercial tumor bespoke Signatera™ assay, was positive for the detection of ctDNA 17 weeks after surgery but prior to local radiotherapy treatment. The ctDNA was cleared after radiation therapy to the breast, which suggested that radiation therapy eliminated residual breast cancer cells, a possibility which is supported by multiple randomized clinical trials (30,31). Pre-operative breast imaging including MRI was unable to detect suspicious breast disease beyond the target cancer. To the best of our knowledge, there have been no previous reports of the clearance of ctDNA due to local radiation therapy for early breast cancer. Several follow up ctDNA tests did not reveal any further ctDNA up to 22 months of follow up. As the patient had LCIS in her breast, it is possible that there were multiple other foci of lobular cancer in her diseased breast, invisible to imaging. These foci may have accounted for the presence of ctDNA in her blood after surgery. Radiotherapy appears to be effective in eliminating this disease, based on the lack of local recurrence during almost 2 years of follow up.
As the present study is limited to a single patient, this alone cannot establish it as a disease-monitoring tool. However, this case demonstrated that bespoke tumor assays have the capacity to detect local subclinical breast cancer. These results provide a rationale for larger prospective studies to establish if ctDNA analysis could be considered an effective decision-making tool in the management of radiation therapy decisions in early-stage breast cancer. In a future study, ctDNA levels should evaluated before and after treatment, including locoregional treatments, if necessary. During the adjuvant period, ctDNA should be compared with traditional blood markers such as CA15.3, CEA and CA125 in order to detect recurrences.
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
The data generated in the present study are not publicly available due to proprietary information but may be requested from the corresponding author. Sequencing data are not available as the Signatera™ test is a commercially available clinical test, with these data not being provided by the company.
Authors' contributions
Conceptualization was performed by MPC, PF and MB. Data acquisition and curation was performed by MPC, PF and MB. PF was involved in discussions about the treatments of the patient. Data analysis and interpretation were performed by MPC and MB. The manuscript was written by MPC and MB. All authors read and approved the final manuscript. MB and MP confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the patient for publication of the details of her medical case.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
ctDNA |
circulating tumor DNA |
|
BC |
breast cancer |
|
ILC |
invasive lobular carcinoma |
|
MRD |
minimal residual disease |
|
MRI |
magnetic resonance imaging |
|
pCR |
pathological complete response |
References
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Mouabbi JA, Hassan A, Lim B, Hortobagyi GN, Tripathy D and Layman RM: Invasive lobular carcinoma: An understudied emergent subtype of breast cancer. Breast Cancer Res Treat. 193:253–264. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Thomas M, Kelly ED, Abraham J and Kruse M: Invasive lobular breast cancer: A review of pathogenesis, diagnosis, management, and future directions of early stage disease. Semin Oncol. 46:121–132. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Wilson N, Ironside A, Diana A and Oikonomidou O: Lobular breast cancer: A review. Front Oncol. 10:5913992021. View Article : Google Scholar : PubMed/NCBI | |
|
Pramod N, Nigam A, Basree M, Mawalkar R, Mehra S, Shinde N, Tozbikian G, Williams N, Majumder S and Ramaswamy B: Comprehensive review of molecular mechanisms and clinical features of invasive lobular cancer. Oncologist. 26:e943–e953. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Biglia N, Maggiorotto F, Liberale V, Bounous VE, Sgro LG, Pecchio S, D'Alonzo M and Ponzone R: Clinical-pathologic features, long term-outcome and surgical treatment in a large series of patients with invasive lobular carcinoma (ILC) and invasive ductal carcinoma (IDC). Eur J Surg Oncol. 39:455–460. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Choi YJ, Shin YD and Song YJ: Comparison of ipsilateral breast tumor recurrence after breast-conserving surgery between ductal carcinoma in situ and invasive breast cancer. World J Surg Oncol. 14:1262016. View Article : Google Scholar : PubMed/NCBI | |
|
Giannakeas V, Sopik V and Narod SA: Association of radiotherapy with survival in women treated for ductal carcinoma in situ with lumpectomy or mastectomy. JAMA Netw Open. 1:e1811002018. View Article : Google Scholar : PubMed/NCBI | |
|
Boyages J and Baker L: Evolution of radiotherapy techniques in breast conservation treatment. Gland Surg. 7:5762018. View Article : Google Scholar : PubMed/NCBI | |
|
Tsai CJ, Huang HY, Chen FM, Yang YH, Chen LC and Hsieh KP: Investigating the effectiveness of adjuvant therapy for patients with hormone receptor-positive ductal carcinoma in situ. PLoS One. 17:e02629342022. View Article : Google Scholar : PubMed/NCBI | |
|
Mills MN, Russo NW, Fahey M, Nanda RH, Raiker S, Jastrzebski J, Stout LL, Wilson JP, Altoos TA, Allen KG, et al: Increased risk for ipsilateral breast tumor recurrence in invasive lobular carcinoma after accelerated partial breast irradiation brachytherapy. Oncologist. 26:e1931–e1938. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Sanz-Garcia E, Zhao E, Bratman SV and Siu LL: Monitoring and adapting cancer treatment using circulating tumor DNA kinetics: Current research, opportunities, and challenges. Sci Adv. 8:eabi86182022. View Article : Google Scholar : PubMed/NCBI | |
|
Zavarykina TM, Lomskova PK, Pronina IV, Khokhlova SV, Stenina MB and Sukhikh GT: Circulating tumor DNA is a variant of liquid biopsy with predictive and prognostic clinical value in breast cancer patients. Int J Mol Sci. 24:170732023. View Article : Google Scholar : PubMed/NCBI | |
|
Arisi MF, Dotan E and Fernandez SV: Circulating tumor DNA in precision oncology and its applications in colorectal cancer. Int J Mol Sci. 23:44412022. View Article : Google Scholar : PubMed/NCBI | |
|
Reinert T, Henriksen TV, Christensen E, Sharma S, Salari R, Sethi H, Knudsen M, Nordentoft I, Wu HT, Tin AS, et al: Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol. 5:1124–1131. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bertlett JMS, Bilous M, Fitzgibbons P, et al: Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 31:3997–4013. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
D'Orsi C, Bassett L and Feig S: Breast imaging reporting and data system (BI-RADS). Breast Imaging Atlas. 4th edition. American College of Radiology; Reston, VA, USA: pp. 29–47. 2018 | |
|
Amin MD, Gress DM, Meyer Vega LR, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK and Compton CC; American Joint Committee on Cancer (AJCC), : AJCC cancer staging manual. 8th edition. Springer; New York, NY, USA: pp. 10322017 | |
|
Olsson E, Winter C, George A, Chen Y, Howlin J, Tang MH, Dahlgren M, Schulz R, Grabau D and van Westen D: Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol Med. 7:1034–1047. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Vlataki K, Antonouli S, Kalyvioti C, Lampri E, Kamina S, Mauri D, Harissis HV and Magklara A: Circulating tumor DNA in the management of early-stage breast cancer. Cells. 12:15732023. View Article : Google Scholar : PubMed/NCBI | |
|
Magbanua MJM, Swigart LB, Wu HT, Hirst GL, Yau C, Wolf DM, Tin A, Salari R, Shchegrova S, Pawar H, et al: Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol. 32:229–239. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Fiste O, Liontos M, Koutsoukos K, Terpos E, Dimopoulos MA and Zagouri F: Circulating tumor DNA-based predictive biomarkers in breast cancer clinical trials: A narrative review. Ann Transl Med. 8:16032020. View Article : Google Scholar : PubMed/NCBI | |
|
Adejolu M, Huo L, Rohren E, Santiago L and Yang WT: False-positive lesions mimicking breast cancer on FDG PET and PET/CT. AJR Am J Roentgenol. 198:W304–W314. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Garcia-Murillas I, Schiavon G, Weigelt B, Ng C, Hrebien S, Cutts RJ, Cheang M, Osin P, Nerurkar A, Kozarewa I, et al: Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 7:302ra1332015. View Article : Google Scholar : PubMed/NCBI | |
|
Lipsyc-Sharf M, de Bruin EC, Santos K, McEwen R, Stetson D, Patel A, Kirkner GJ, Hughes ME, Tolaney SM, Partridge AH, et al: Circulating tumor DNA and late recurrence in high-risk hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer. J Clin Oncol. 40:2408–2419. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Magbanua MJM, van't Veer L, Clark AS, Chien AJ, Boughey JC, Han HS, Wallace A, Beckwith H, Liu MC, Yau C, et al: Outcomes and clinicopathologic characteristics associated with disseminated tumor cells in bone marrow after neoadjuvant chemotherapy in high-risk early stage breast cancer: The I-SPY SURMOUNT study. Breast Cancer Res Treat. 198:383–390. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Cavallone L, Aguilar-Mahecha A, Lafleur J, Brousse S, Aldamry M, Roseshter T, Lan C, Alirezaie N, Bareke E, Majewski J, et al: Prognostic and predictive value of circulating tumor DNA during neoadjuvant chemotherapy for triple negative breast cancer. Sci Rep. 10:147042020. View Article : Google Scholar : PubMed/NCBI | |
|
Coombes RC, Page K, Salari R, Hastings RK, Armstrong A, Ahmed S, Ali S, Cleator S, Kenny L, Stebbing J, et al: Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res. 25:4255–4263. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Cailleux F, Agostinetto E, Lambertini M, Rothé F, Wu HT, Balcioglu M, Kalashnikova E, Vincent D, Viglietti G, Gombos A, et al: Circulating tumor DNA after neoadjuvant chemotherapy in breast cancer is associated with disease relapse. JCO Precis Oncol. 6:e22001482022. View Article : Google Scholar : PubMed/NCBI | |
|
Lin PH, Wang MY, Lo C, Tsai LW, Yen TC, Huang TY, Huang WC, Yang K, Chen CK, Fan SC, et al: Circulating tumor DNA as a predictive marker of recurrence for patients with stage II–III breast cancer treated with neoadjuvant therapy. Front Oncol. 11:7367692021. View Article : Google Scholar : PubMed/NCBI | |
|
Lee TH, Kim H, Kim YJ, Park WY, Park W, Cho WK and Kim N: Implication of pre- and post-radiotherapy ctDNA dynamics in patients with residual triple-negative breast cancer at surgery after neoadjuvant chemotherapy: Findings from a prospective observational study. Cancer Res Treat. Nov 10–2023.(Epub ahead of print). |