Prognostic and diagnostic effects of high serum midkine levels in patients with hepatocellular carcinoma
- Authors:
- Published online on: April 25, 2024 https://doi.org/10.3892/ol.2024.14416
- Article Number: 283
Abstract
Introduction
Midkine (MK) is a pleiotropic growth binding protein that is highly upregulated during embryogenesis, thereby playing a key role in neuronal differentiation (1,2). Furthermore, MK exhibits antiapoptotic and angiogenic activities and can lead to enhanced cell proliferation in tumors. Since MK is a soluble cytokine, its serum levels strongly correspond to protein expression levels in tumors (3). Serum MK (s-MK) has been proposed as a potential biomarker for different tumors, including hepatocellular carcinoma (HCC).
Serum α-fetoprotein (AFP) is the only diagnostic marker recommended in the HCC guidelines. However, its diagnostic performance is unsatisfactory, with low sensitivity and specificity. To improve the diagnosis of HCC, advances in biomarker detection techniques have led to the identification of several new biomarkers, such as autoantibodies and s-MK (4–6). s-MK, an emerging serum biomarker, activates several cell surface receptors to modulate various biological activities and is significantly increased in HCC (7). s-MK has been proposed as a promising serum biomarker for HCC diagnosis. Although several studies have estimated the diagnostic value of s-MK for HCC, the results are inconsistent (8–12). Precise clinicopathological analyses including AFP and protein induced by vitamin K absence-II (PIVKA-II) have not been published.
An s-MK-positive status has been reported to be associated with poor prognosis in some solid tumors, such as colorectal cancer and non-small cell lung cancer, but not in esophageal and gastric cancers (13–16). The correlation between an s-MK-positive status and prognosis of patients with HCC has not been published.
Therefore, this study aimed to clarify the clinicopathological and prognostic significance of an s-MK-positive status in patients with HCC.
Materials and methods
Patients
This study was registered as UMIN000014530. Serum samples were obtained before surgery from 123 patients with HCC who had undergone surgery at Omori Medical Center, Toho University School of Medicine, between January 2012 and December 2020. In total, 123 patients with histologically proven primary HCC were enrolled. The patient cohort consisted of 87 male (70.7%) and 36 female (29.3%) patients, with a median age of 69 (range, 40–85) years. To ensure complete absence of the influence of previous cancer, those with active coexisting cancer, i.e., synchronous coexisting cancer or metachronous cancer within 5 disease-free years, were excluded. The final HCC stage was assessed pathologically following the tumor-node-metastasis classification criteria of the eighth edition of the International Union against Cancer (17). Tumors associated with distant metastasis, including peritoneal dissemination, were considered unresectable. Hepatectomy was performed according to the treatment algorithm described in Japanese guidelines (18,19). The degree of liver damage is defined by the following factors: Ascites, serum total bilirubin level, serum albumin level, ICG R15, prothrombin activity value (20).
Data collection and serum biomarker analyses
Serum samples were obtained before surgery and stored at −80°C until analysis. Serum samples of healthy controls, with no previous malignant disease and hepatitis B or C infection, were obtained from Biobank Japan. The average age of the control group (n=77) was 52 years, with a male-to-female ratio of 50:27.
Clinicopathological characteristics, AFP, and PIVKA-II were analyzed. Preoperative variables, pathological characteristics, postoperative status, and survival were entered into a spreadsheet and imported to a dedicated database. The prognostic value and clinical utility of s-MK for HCC diagnosis were estimated. Overall survival was calculated from the time of surgery until death or study conclusion.
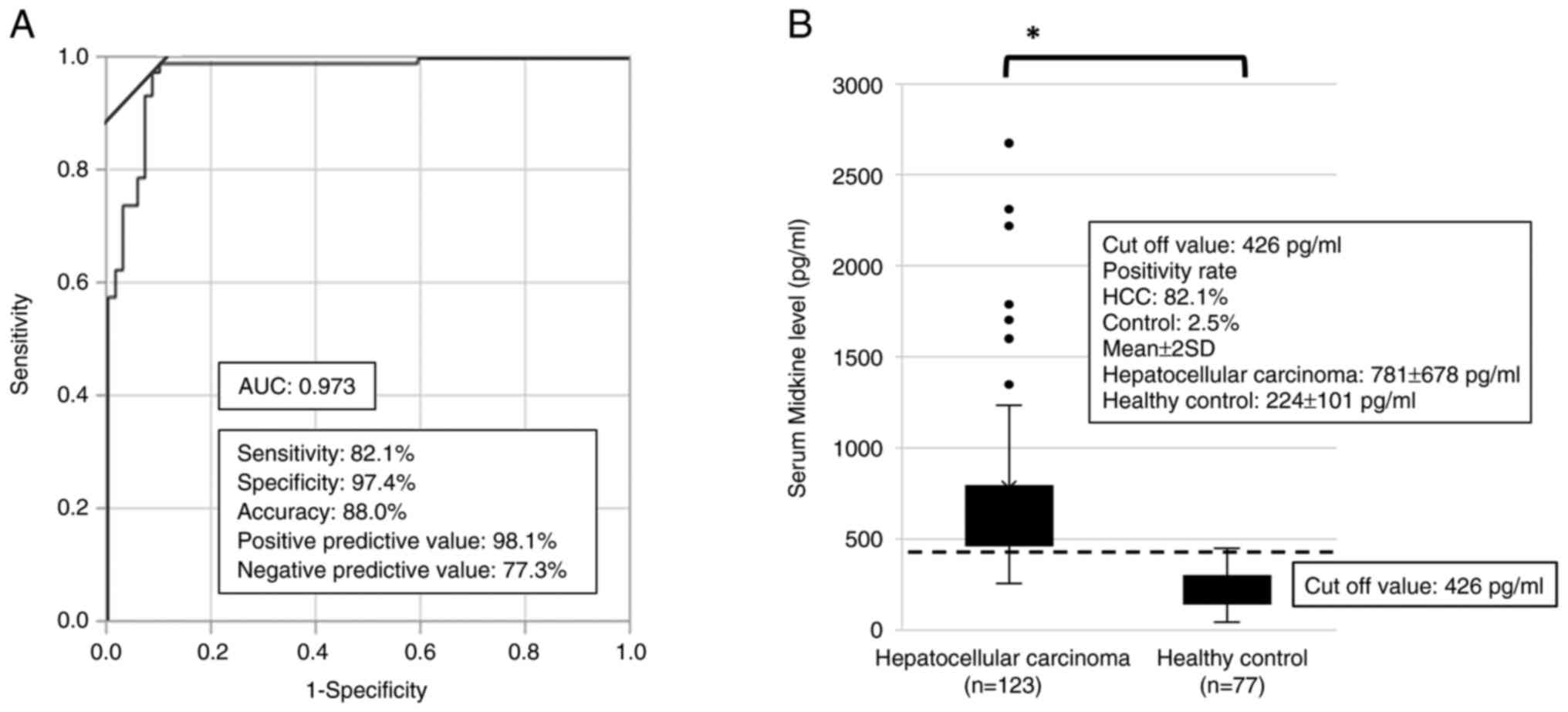
Enzyme-linked immunosorbent assay kits for human MK (CDYELISA, Immuno-probe Ltd., Saitama, Japan) were used for detecting s-MK according to the manufacturer's protocol. The cutoff value for s-MK was fixed at 426 pg/ml based on the receiver operating characteristic curve (Fig. 1A).
Patients' clinicopathological variables, demographics, tumor characteristics, and overall survival were compared between the s-MK-positive group and s-MK-negative group. The cutoff values were 10.0 ng/ml and 40.0 mAU/ml for AFP and PIVKA-II, respectively, following the assay kit manufacturer's instructions.
Statistical analysis
Statistical analyses were performed using JMP version 12 (SAS Institute, Cary, NC, USA). The comparison of s-MK levels in the HCC and healthy control groups was performed using unpaired t-test. A multiple comparison test of ANOVA was performed to compare the positivity rates of s-MK, AFP, and PIVKA-II according to TNM stages. We selected the Bonferroni post hoc test as multiple comparison test. Between-group comparisons of the clinicopathological variables were performed using Fisher's exact probability test. Overall survival was calculated using the Kaplan-Meier product limit estimate. Between-group differences in survival were compared using the log-rank test. Significant predictors were identified via univariate and multivariate analyses using Cox proportional hazard models, and hazard ratios with 95% confidence intervals (CIs) were calculated. A P value of <0.05 was considered statistically significant.
Results
Sensitivity and specificity of serum MK levels
Based on the ROC curve, the best cutoff point was determined to distinguish the HCC group using s-MK. The area under the curve for s-MK was 0.973 (95% CI 0.903–0.992) (Fig. 1A). According to the curve, the best cutoff value for s-MK in differentiating HCC from healthy cases was 426 pg/ml. At this value, the sensitivity, specificity, and accuracy were 82, 97, and 88%, respectively. The mean s-MK levels in the HCC and healthy control groups were 781±678 and 224±101 pg/ml, respectively (Fig. 1B, P<0.05).
Comparison of clinicopathological characteristics between the s-MK-positive group and s-MK-negative group
Of the 123 patients enrolled, 101 (82%) were positive for s-MK (>426 pg/ml) (Table I). An s-MK-positive status was significantly associated with hepatitis B virus negativity and number of tumors (≥2) but not with the liver reserve or liver background.
Table I.Comparisons between serum midkine level according to clinicopathological factors and various biomarkers. |
Positivity rates of s-MK, AFP, and PIVKA-II according to TNM stages
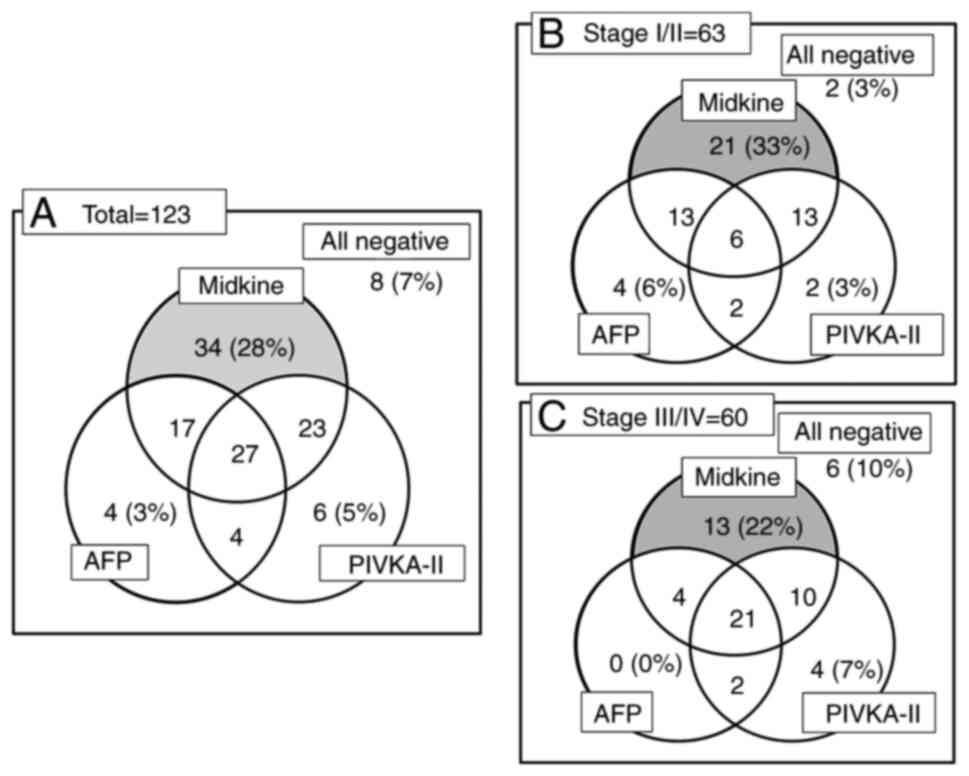
The positivity rates of s-MK were significantly higher than those of AFP and PIVKA-II (P<0.05, Fig. 2A). In total, only 28% (34 of 123) of the patients were positive for s-MK. Among patients with stage I/II, only 33% (21 of 63) were positive for s-MK (Fig. 2B). Even among patients with stage III/IV, only 22% (13 of 60) were positive for s-MK (Fig. 2C).
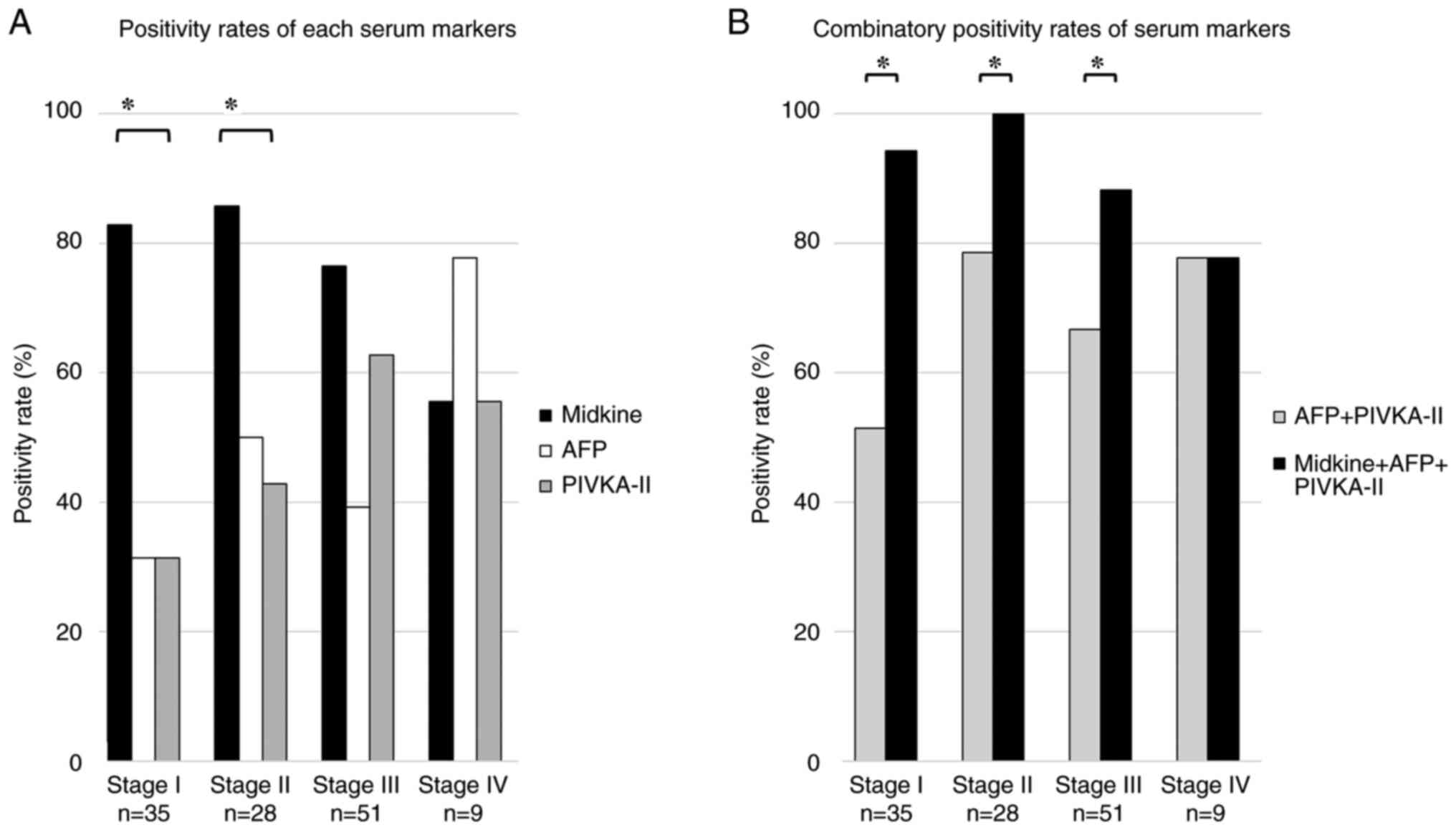
Fig. 3A shows the positivity rates for s-MK, AFP, and PIVKA-II at each TNM stage. In stage I, the positivity rate for s-MK was significantly higher than that for AFP and PIVKA-II (83% vs. 31% vs. 31%, P<0.05). In stage II, the positivity rates for s-MK, AFP, and PIVKA-II were 86, 50, and 43%, respectively (P<0.05). In stage III, the positivity rates for s-MK, AFP, and PIVKA-II were 76, 39, and 63%, respectively (not significant). In stage IV, the positivity rates for s-MK, AFP, and PIVKA-II were 56, 78, and 56% (not significant), respectively.
The positivity rate for the combined use of s-MK and AFP + PIVKA-II was significantly higher than that for AFP + PIVKA-II (93% vs. 65%, P<0.05, Fig. 3B). In stage I, the positivity rate for the combined use of s-MK and AFP + PIVKA-II was significantly higher than that for AFP + PIVKA-II (94% vs. 51%, P<0.05). Moreover, in stage II, the positivity rate for the combined use of s-MK and AFP + PIVKA-II was significantly higher than that for AFP + PIVKA-II (100% vs. 79%, P<0.05). In stage III, the positivity rate for the combined use of s-MK and AFP + PIVKA-II was significantly higher than that for AFP + PIVKA-II (88% vs. 67%, P<0.05).
Prognostic effect of s-MK, AFP, and PIVKA-II status on overall survival
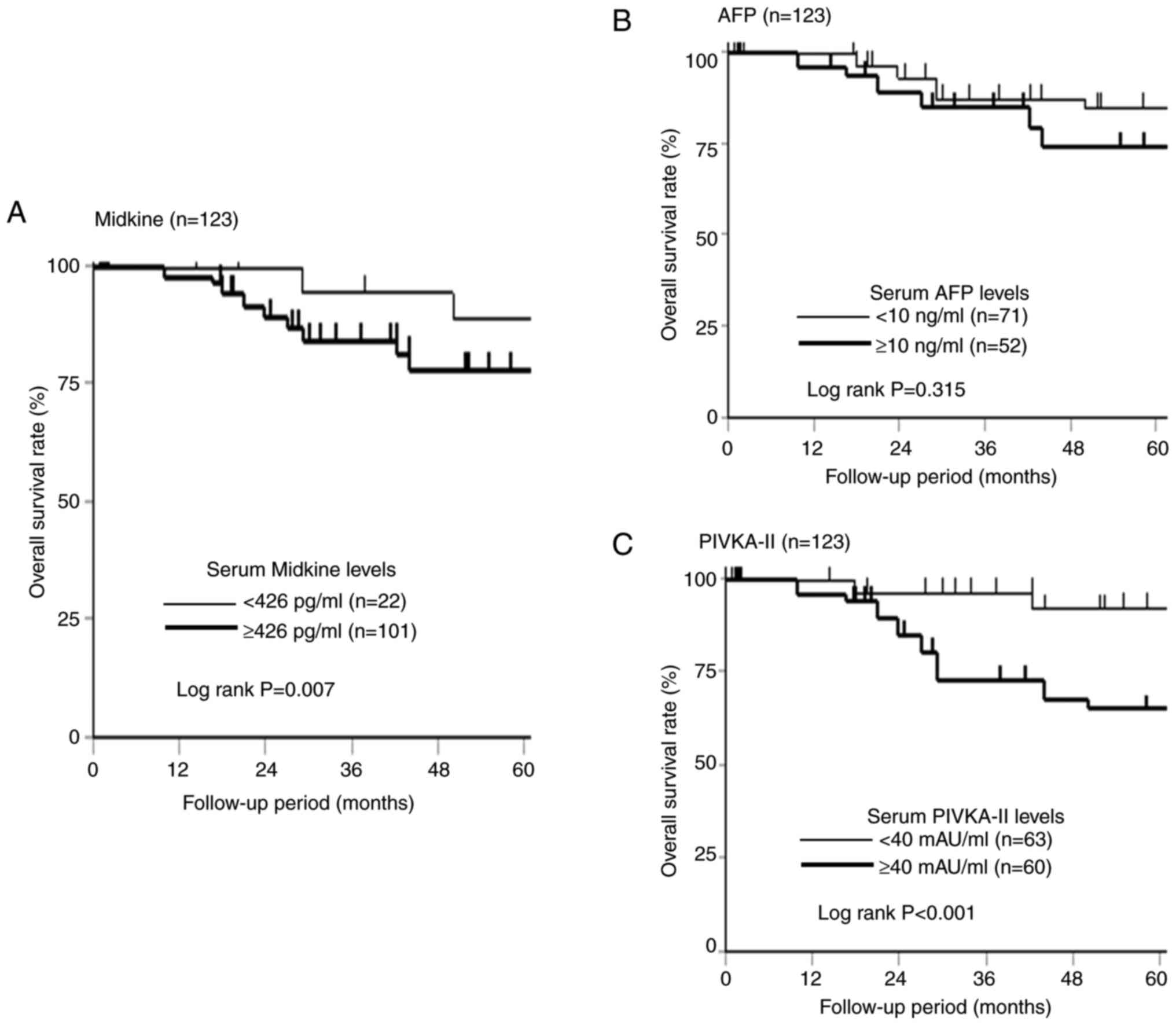
The 5-year overall survival according to the s-MK, AFP, and PIVKA-II status is shown in Fig. 4. Although no significant difference was observed in the overall survival according to the AFP status (Fig. 4B, P=0.315), the s-MK-positive group showed significantly worse overall survival than the s-MK-negative group (Fig. 4A, P=0.007). Similarly, the PIVKA-II-positive group showed significantly poorer overall survival than the PIVKA-II-negative group (Fig. 4C, P<0.001).
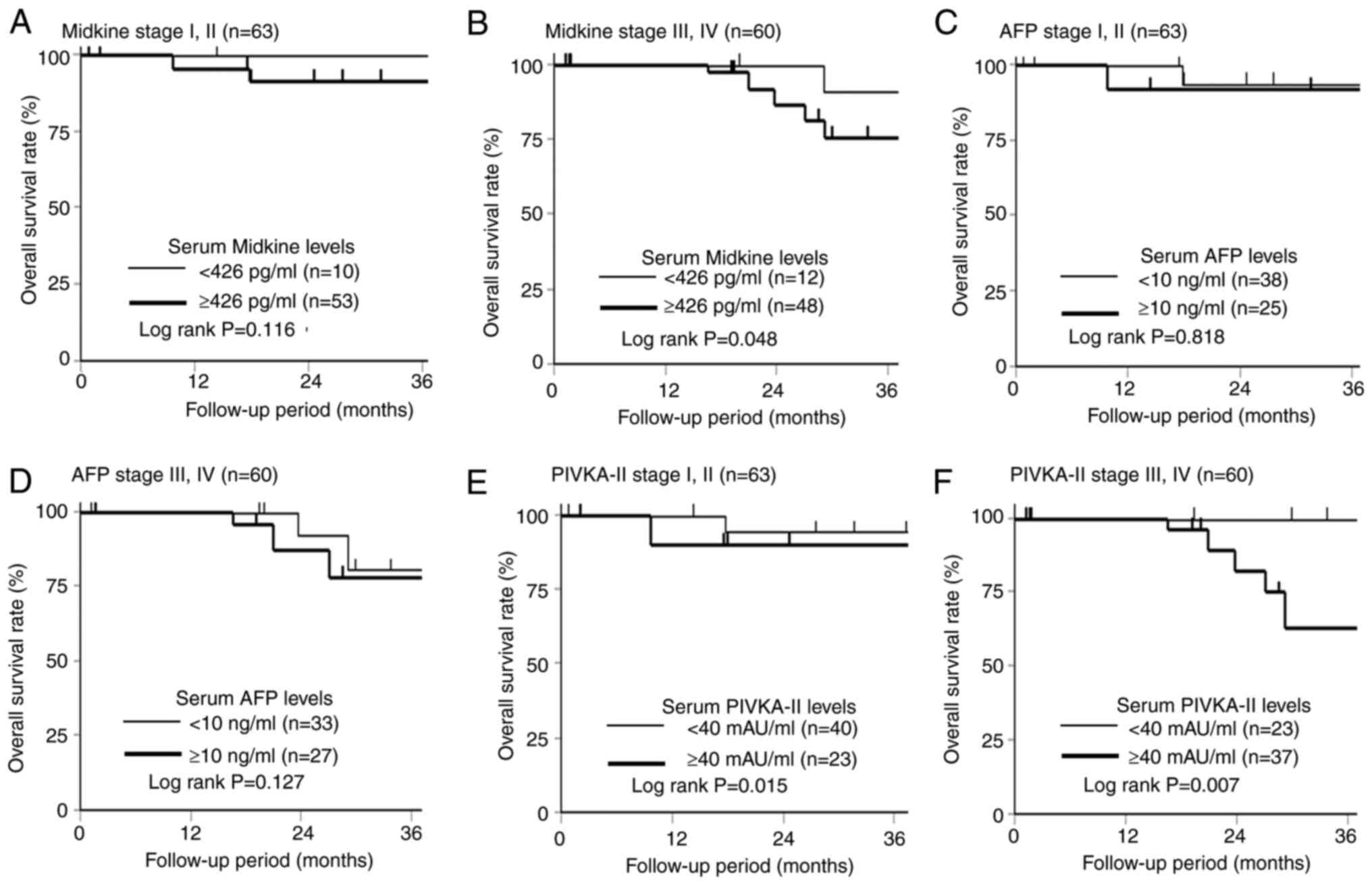
Fig. 5 shows the comparison of overall survival at stages I/II and III/IV according to the s-MK, AFP, and PIVKA-II status. Regarding the prognostic effect of the s-MK status, the s-MK-positive group in stage I/II showed slightly worse overall survival than the s-MK-negative group (Fig. 5A, P=0.116). The s-MK-positive group in stage III/IV showed significantly worse overall survival than the s-MK-negative group (Fig. 5B, P=0.048). No significant difference was observed in the overall survival according to the AFP status (Fig. 5C and D, P=0.818, P=0.127). In contrast, a significant difference was observed in overall survival according to the PIVKA-II status (Fig. 5E and F, P=0.015, P=0.007).
Recurrence effect of s-MK status on recurrence-free survival
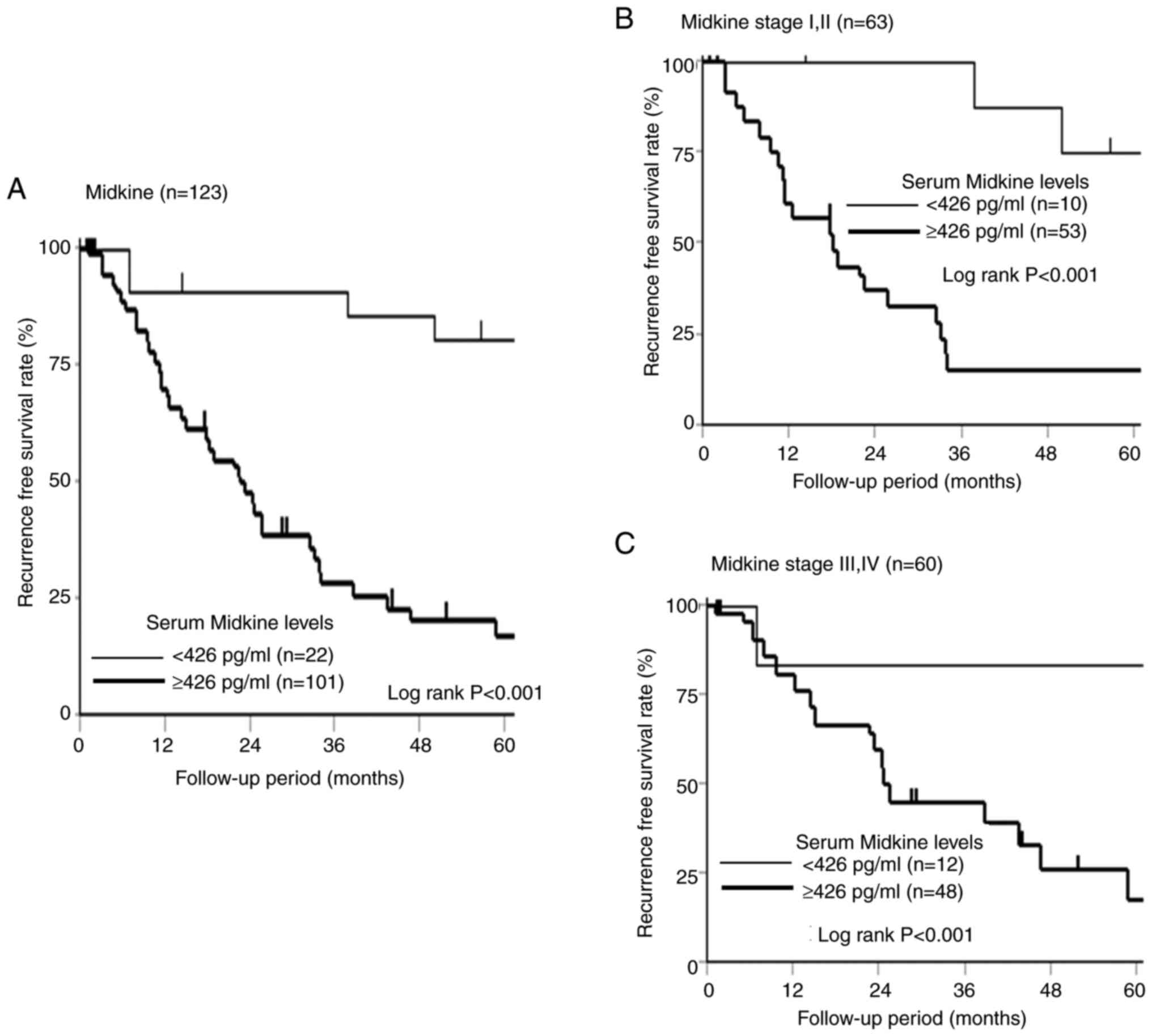
The 5-year recurrence-free survival according to the s-MK status is shown in Fig. 6. The s-MK-positive group showed significantly worse recurrence-free survival than the s-MK-negative group (Fig. 6A, P<0.001). The s-MK-positive group in stage I/II and III/IV showed significantly worse recurrence-free survival than the s-MK-negative group (Fig. 6B and C, P<0.001).
Univariate and multivariate analyses of overall survival
In the univariate analysis, the Child-Pugh classification (B), liver damage (B), PIVKA-II-positive status, and s-MK-positive status were significantly associated with poor prognosis (Table II). In the multivariate analysis, PIVKA-II-positive status (P=0.002; HR=3.759; 95% CI 1.600–9.603) and s-MK-positive status (P=0.006; HR=5.157; 95% CI 1.483–32.553) were independently associated with poor prognosis.
Discussion
The positivity rate for s-MK was 82% in patients with HCC. The positivity rate for the combined use of s-MK and AFP + PIVKA-II was significantly higher than that for AFP + PIVKA-II. An s-MK-positive status was associated with the number of tumors. The s-MK-positive group showed poor overall survival.
An s-MK-positive rate was not associated with stage, and this tendency was similar to the pattern of serum autoantibodies, as previously reported (5,6). s-MK is induced not only by cancer but also by various factors such as inflammation and hemodynamics (21). At present, even in HCC, which has multistage carcinogenesis, the stage at which s-MK is induced is unclear. Shaheen et al reported that the s-MK level was significantly elevated in the HCC group compared with the healthy control group and liver cirrhosis group (22). These findings suggest that s-MK can be used to detect early-stage cancer follow up patients with cirrhosis.
In the present study, s-MK was associated with the number of tumors but not with liver background or tumor size. Among the 23 patients with multiple tumors, the positivity rates for s-MK, AFP, and PIVKA-II were 100, 69, and 43%, respectively. This may be because MK plays an important role in cell proliferation, survival, migration, angiogenesis, and carcinogenesis (23,24). Whether s-MK is a cause or a consequence of multiple tumors is unclear. However, given that an s-MK-positive status is a poor prognostic factor, an s-MK-positive status may reflect the biological grade of the tumor.
The prognostic effect of s-MK on various cancers was not consistent. In this study, we first evaluated the prognostic effect of s-MK on HCC. An s-MK-positive status was an independent risk factor for poor overall survival. The poor prognostic effect of an s-MK-positive status in HCC suggests the high biological malignancy of s-MK-positive HCC cells, given the lack of correlation between an s-MK-positive status and cirrhosis. MK-positive cancer cells have been reported to be associated with antiapoptotic function, and resistance to chemotherapy after HCC recurrence may contribute to poor prognosis (25). Considering that miRNA519d, an exosome derived from HCC, can inhibit apoptosis and distinguish between cirrhotic patients without HCC and cirrhotic patients with early-stage HCC, miRNA519d and s-MK may have a common mechanism (26). Considering the results of the IMbrave050 trial, patients with an s-MK positive status who are at a high risk of recurrence may be able to effectively prolong their recurrence-free survival by receiving adjuvant atezolizumab plus bevacizumab (27).
This study had some limitations. First, the sample size was not large enough. Assuming a 95% confidence level and a 5% confidence interval, we were unable to collect a sample size large enough for this study. Second, no data were available for evaluating the association between s-MK positivity and the immunoreactivity of cancer cells. Since several previous studies have reported that s-MK concentrations are significantly associated with immunoreactivity, MK expression in cancer cells may similarly be associated with s-MK (28,29). Third, we did not analyze the other cytokines, such as serum vascular endothelial growth factor (VEGF), in this study. Alzamzamy et al reported that in patients with HCV, serum VEGF and VEGF/PLT separately or in combination with AFP are reliable biomarkers for early and accurate HCC diagnosis (30). Furthermore, Mamdouh et al reported that the serum VEGF levels in patients with HCC and cirrhosis were significant compared with the control group (31). It is possible that s-MK, together with cytokines such as VEGF, will play a major role in the diagnosis of hepatocellular carcinoma in the future. Fourth, this study only focused on preoperative s-MK and had no data of postoperative monitoring. Therefore, we could not capture changes in s-MK levels before and after surgery. The s-MK level was reported to decrease significantly after surgery in esophageal cancer (28).
In conclusion, s-MK was a convenient and useful serum biomarker to detect HCC even in patients with stage I/II regardless of LC. An s-MK-positive status was associated with the number of tumors and was an independent prognostic risk factor. Considering the malignant potential of s-MK-positive HCC, more intensive follow-up is necessary after surgery.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
RO and HS confirmed the authenticity of all the raw data. RO conceptualized and designed the study, performed the statistical analysis and prepared the manuscript. YO, YK, TM, JI, KK, YM, YI and KF acquired the data. RO and YO performed the quality control of data and algorithms. RO, YO and HS analyzed and interpreted the data. RO and HS edited the manuscript. All authors reviewed the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
All study participants provided consent for future analyses of their blood samples for research. The protocol for this study was approved by the Ethics Committee of Toho University (approval nos. M22211, M21038_20197_19213 and A18103_A17052_A16035_A16001_26095_25024_24038_22047_22112). Patients provided written informed consent before enrolment. The study was registered in the UMIN Clinical Trials Registry (clinical trial no. UMIN000014530) and was conducted following the guidelines of the Declaration of Helsinki and the Japanese Ethical Guidelines for Clinical Research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
AFP |
α-fetoprotein |
|
HCC |
hepatocellular carcinoma |
|
MK |
midkine |
|
PIVKA-II |
protein-induced by vitamin K absence-II |
References
|
Kadomatsu K: Midkine, a heparin-binding growth factor: Its discovery and functions. Seikagaku. 70:1315–1325. 1998.(In Japanese). PubMed/NCBI | |
|
Muramatsu T: Midkine, a heparin-binding cytokine with multiple roles in development, repair and diseases. Proc Jpn Acad Ser B Phys Biol Sci. 86:410–425. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Jones DR: Measuring midkine: The utility of midkine as a biomarker in cancer and other diseases. Br J Pharmacol. 171:2925–2939. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y and Nakatsura T: Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 21:10573–10583. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Okada R, Otsuka Y, Wakabayashi T, Shinoda M, Aoki T, Murakami M, Arizumi S, Yamamoto M, Aramaki O, Takayama T, et al: Six autoantibodies as potential serum biomarkers of hepatocellular carcinoma: A prospective multicenter study. Int J Cancer. 147:2578–2586. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Okada R, Otsuka Y, Yokosuka O, Kato N, Imazaki F, Hoshino I, Sugiura N, Mizumoto H, Azemoto R, Kato K and Shimada H: Six autoantibodies as potential differential biomarkers of hepatocellular carcinoma vs. liver cirrhosis and chronic hepatitis: A prospective multi-institutional study. Oncol Lett. 24:3672022. View Article : Google Scholar : PubMed/NCBI | |
|
Kadomatsu K and Muramatsu T: Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 204:127–143. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Mashaly AH, Anwar R, Ebrahim MA, Eissa LA and El Shishtawy MM: Diagnostic and prognostic value of talin-1 and midkine as tumor markers in hepatocellular carcinoma in Egyptian patients. Asian Pac J Cancer Prev. 19:1503–1508. 2018.PubMed/NCBI | |
|
Hodeib H, ELshora O, Selim A, Sabry NM and El-Ashry HM: Serum midkine and osteopontin levels as diagnostic biomarkers of hepatocellular carcinoma. Electron Physician. 9:3492–3498. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Vongsuvanh R, van der Poorten D, Iseli T, Strasser SI, McCaughan GW and George J: Midkine increases diagnostic yield in AFP negative and NASH-related hepatocellular carcinoma. PLoS One. 11:e01558002016. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang BH, Li B, Kong LX, Yan LN and Yang JY: Diagnostic accuracy of midkine on hepatocellular carcinoma: A meta-analysis. PLoS One. 14:e02235142019. View Article : Google Scholar : PubMed/NCBI | |
|
Lu Q, Li J, Cao H, Lv C, Wang X and Cao S: Comparison of diagnostic accuracy of midkine and AFP for detecting hepatocellular carcinoma: A systematic review and meta-analysis. Biosci Rep. 40:BSR201924242020. View Article : Google Scholar : PubMed/NCBI | |
|
Kemper M, Hentschel W, Graß JK, Stüben BO, Konczalla L, Rawnaq T, Ghadban T, Izbicki JR and Reeh M: Serum midkine is a clinical significant biomarker for colorectal cancer and associated with poor survival. Cancer Med. 9:2010–2018. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Stern L, Mueller E, Bellon E, Reeh M, Grotelueschen R, Guengoer C, Melling N, Goetz M, Perez DR, Izbicki JR, et al: Serum midkine as non-invasive biomarker for detection and prognosis of non-small cell lung cancer. Sci Rep. 11:146162021. View Article : Google Scholar : PubMed/NCBI | |
|
Shiratori F, Ito M, Yajima S, Suzuki T, Oshima Y, Nanami T, Funahashi K and Shimada H: The effectiveness of serum midkine in detecting esophageal squamous cell carcinoma. Esophagus. 16:246–251. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ito M, Oshima Y, Yajima S, Suzuki T, Nanami T, Shiratori F, Funahashi K and Shimada H: Diagnostic impact of high serum midkine level in patients with gastric cancer. Ann Gastroenterol Surg. 3:195–201. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Union for International Cancer Control, . TNM Classification of Malignant Tumors. Brierley JD, Gospodarowicz MK and Wittekind CH: 8th edition. UICC; Wiley, New York, NY: 2001 | |
|
Makuuchi M and Kokudo N: Clinical practice guidelines for hepatocellular carcinoma: The first evidence based guidelines from Japan. World J Gastroenterol. 12:828–829. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y and Takayama T: Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 26:1176–1181. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M and Makuuchi M; HCC Expert Panel of Japan Society of Hepatology, : Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 29:339–364. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Ketenci S, Uygar Kalaycı M, Dündar B, Duranay R and Şükrü Aynacıoğlu A: Elevated serum midkine levels in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected patients. Int Immunopharmacol. 110:1089392022. View Article : Google Scholar : PubMed/NCBI | |
|
Shaheen KY, Abdel-Mageed AI, Safwat E and AlBreedy AM: The value of serum midkine level in diagnosis of hepatocellular carcinoma. Int J Hepatol. 2015:1463892015. View Article : Google Scholar : PubMed/NCBI | |
|
Shin DH, Jo JY, Kim SH, Choi M, Han C, Choi BK and Kim SS: Midkine is a potential therapeutic target of tumorigenesis, angiogenesis, and metastasis in non-small cell lung cancer. Cancers (Basel). 12:24022020. View Article : Google Scholar : PubMed/NCBI | |
|
Karadeniz Z, Aynacıoğlu AŞ, Bilir A and Tuna MY: Inhibition of midkine by metformin can contribute to its anticancer effects in malignancies: A proposal mechanism of action of metformin in context of endometrial cancer prevention and therapy. Med Hypotheses. 134:1094202020. View Article : Google Scholar : PubMed/NCBI | |
|
Qi M, Ikematsu S, Ichihara-Tanaka K, Sakuma S, Muramatsu T and Kadomatsu K: Midkine rescues Wilms' tumor cells from cisplatin-induced apoptosis: Regulation of Bcl-2 expression by midkine. J Biochem. 127:269–277. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Sasaki R, Kanda T, Yokosuka O, Kato N, Matsuoka S and Moriyama M: Exosomes and hepatocellular carcinoma: From bench to bedside. Int J Mol Sci. 20:14062019. View Article : Google Scholar : PubMed/NCBI | |
|
Qin S, Chen M, Cheng AL, Kaseb AO, Kudo M, Lee HC, Yopp AC, Zhou J, Wang L, Wen X, et al: Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): A randomised, open-label, multicentre, phase 3 trial. Lancet. 402:1835–1847. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Yamashita T, Shimada H, Tanaka S, Araki K, Tomifuji M, Mizokami D, Tanaka N, Kamide D, Miyagawa Y, Suzuki H, et al: Serum midkine as a biomarker for malignancy, prognosis, and chemosensitivity in head and neck squamous cell carcinoma. Cancer Med. 5:415–425. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Shimada H, Nabeya Y, Tagawa M, Okazumi S, Matsubara H, Kadomatsu K, Muramatsu T, Ikematsu S, Sakuma S and Ochiai T: Preoperative serum midkine concentration is a prognostic marker for esophageal squamous cell carcinoma. Cancer Sci. 94:628–632. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Alzamzamy A, Elsayed H, Abd Elraouf M, Eltoukhy H, Megahed T and Aboubakr A: Serum vascular endothelial growth factor as a tumor marker for hepatocellular carcinoma in hepatitis C virus-related cirrhotic patients. World J Gastrointest Oncol. 13:600–611. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Mamdouh S, Soliman A, Khorshed F and Saber M: Glypican-3, vascular endothelial growth factor and golgi protein-73 for differentiation between liver cirrhosis and hepatocellular carcinoma. Asian Pac J Cancer Prev. 24:497–507. 2023. View Article : Google Scholar : PubMed/NCBI |