A propensity‑matched analysis of the prognostic value of advanced lung cancer inflammation index in patients with gastric cancer after curative resection
- Authors:
- Published online on: April 26, 2024 https://doi.org/10.3892/ol.2024.14418
- Article Number: 285
-
Copyright: © Hashimoto et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Gastric cancer (GC) is the third leading cause of cancer-related mortality globally and is identified as the fifth most prevalent malignancy in the world (1). Despite significant advancements in GC management encompassing endoscopy, surgical interventions, and chemotherapy, treatment outcomes remain to be significantly improved (2). In recent years, the perioperative inflammatory and nutritional status of patients has garnered increased attention for its potential impact on the treatment outcomes and overall prognosis of cancer (3–9). Several methodologies have been developed for the assessment of inflammation and nutritional status in patients with GC (10–12). Nonetheless, further research to refine and develop more effective indices for the assessment of patients with GC is warranted.
The Advanced Lung Cancer Inflammation Index (ALI), conceptualized by Jafri et al, is a novel prognostic predictor for patients with metastatic non-small cell lung cancer (13). ALI combines the Neutrophil-to-Lymphocyte Ratio/Albumin (NLR/Alb) ratio (14), which reflects inflammation, nutritional, and immune status, with the body mass index (BMI) (15), a straightforward metric of obesity. The prognostic value of ALI has been documented across various cancer types (16–18). However, the applicability of ALI as a prognostic tool specifically for patients with GC has been sporadically reported (19–23) and remains insufficiently explored. Consequently, in this study, we aimed to evaluate the clinical significance of preoperative ALI measurement in patients with GC undergoing curative resection.
Materials and methods
Patients
A total of 459 patients with GC were enrolled in this study at the Kanagawa Cancer Center between December 2013 and November 2017. The inclusion criteria were as follows: i) GC confirmed by pathological diagnosis; ii) gastrectomy achieving R0 resection with radical lymph node resection as the initial treatment for GC; iii) age over 20 years, and iv) Eastern Cooperative Oncology Group performance status of 0–2. In principle, pathological stage (pStage) II patients received S-1 mono-therapy, and pStage III patients received S-1 therapy plus docetaxel or capecitabine plus oxaliplatin therapy for one year. All study protocols were approved by the Ethics Committee of the Kanagawa Cancer Center (approval number: 25Research-20), and all procedures were conducted following the Declaration of Helsinki in 1996. In this study, informed consent was obtained from all patients by completing an informed consent form.
Measurement of ALI
We calculated ALI based on preoperative blood test data as follows: ALI=BMI (kg/m2) * albumin (g/dl)/[Neutrophil (/µl)/Lymphocyte (/µl)]. The cutoff value was defined as 56.8 based on a receiver operating characteristic analysis of survival and death (area under the curve 0.61, 95% confidence interval 0.54–0.68). According to the cutoff value, the patients were categorized into high- and low-ALI groups.
Analyzed parameters
Prognostic factors were examined using the following variables: patient age, sex, BMI, operation, tumor size, histological type, lymphatic invasion, venous invasion, pathological Stage (pStage), and postoperative complications.
Statistical analyses
Categorical variables were examined using the χ2 test or Fisher exact test, as appropriate. Propensity matched analysis was performed for ALI. The matched variables included age, sex, and lymphatic invasion. Overall survival (OS) and relapse-free survival (RFS) were assessed using the Kaplan-Meier method and log-rank test. Variables identified as significant (P<0.05) in the univariate analysis were considered candidates for the multivariate COX regression analysis, and results were presented as hazard ratios (HRs) with a 95% confidence interval (CI). A P-value <0.05 was considered significant. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Relationship between ALI and clinicopathological factors
Table I shows the relationship between preoperative ALI and clinicopathological factors in patients with GC. A total of 459 patients were categorized into the high-ALI (n=150) and low-ALI (n=309) groups based on their preoperative ALI (Table I). In the low-ALI group, patients were older (P=0.017), more likely to be female (P=0.007), had lower BMI (P<0.001), and had a higher incidence of lymphatic invasion (P=0.03) by comparison with the high-ALI group before propensity matched analysis.
Relationship between ALI and OS and RFS
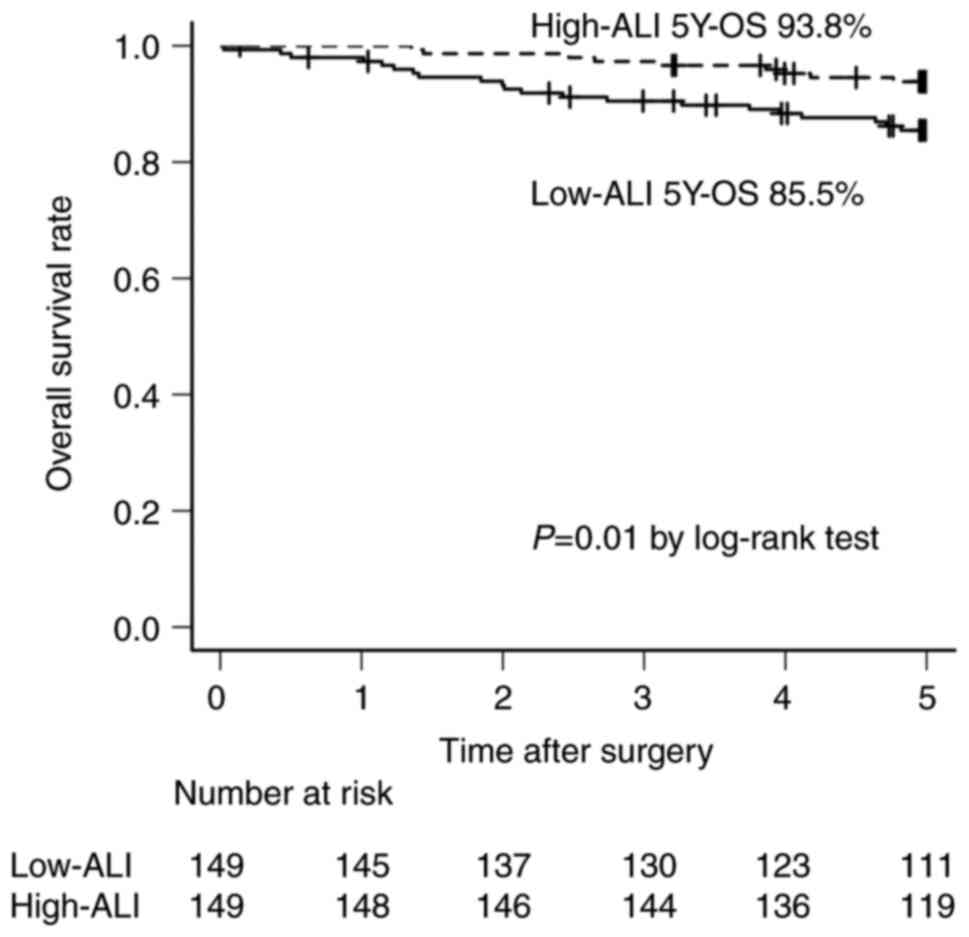
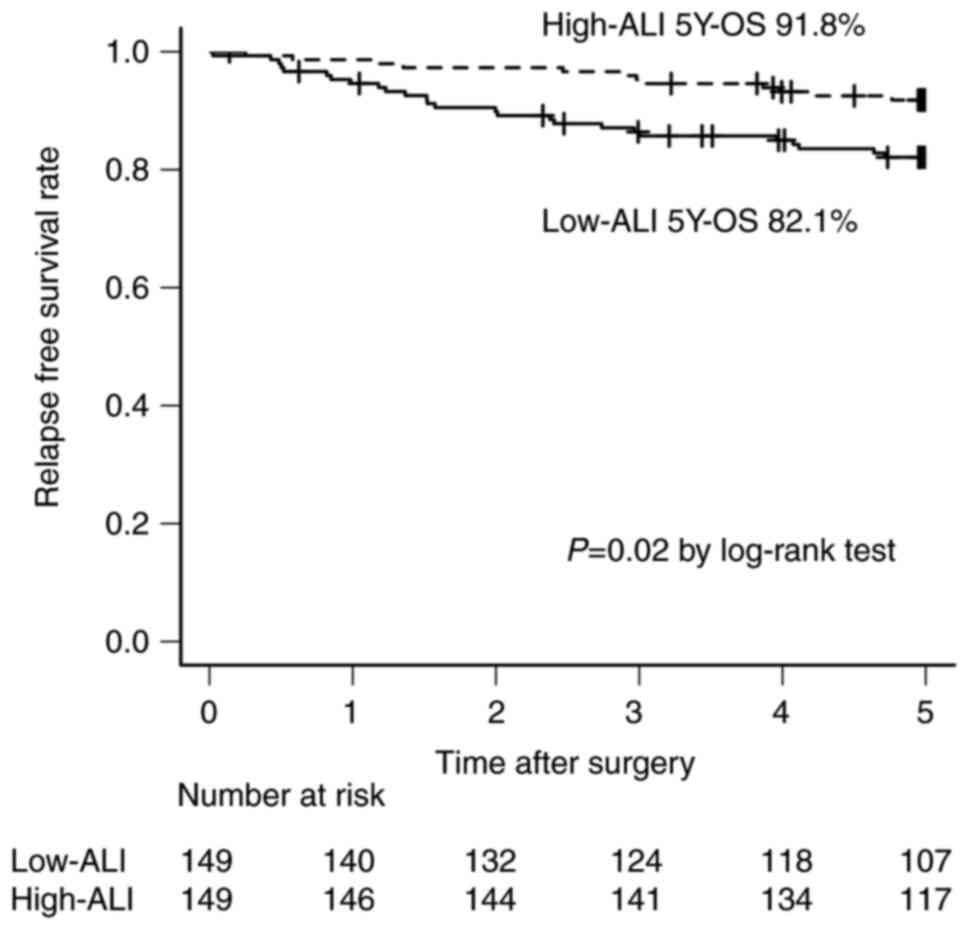
The OS of the low-ALI group was significantly poorer than that of the high-ALI group (85.5% vs. 93.8%, P=0.01) (Fig. 1). The RFS of the low-ALI group was significantly poorer than that of the high-ALI group (82.1% vs. 91.8%, P=0.02) (Fig. 2).
Univariate and multivariate analyses for OS and RFS
Tables II and III show the results of the univariate and multivariate analyses of OS and RFS in patients with GC, who underwent gastrectomy according to ALI. Multivariate analyses for OS demonstrated that low ALI (Hazard Ratio [HR]: 2.26; 95% CI: 1.11–4.59; P=0.03) was an independent prognostic factor (Table II). Moreover, multivariate analyses for RFS demonstrated that age≥65 years (HR: 2.35; 95% CI: 1.15–4.80; P=0.03), lymphatic invasion (HR: 2.08; 95% CI: 1.03–4.18; P=0.04), pStage II/III (HR: 2.13; 95% CI: 1.03–4.41; P=0.04), and low ALI (HR: 1.97; 95% CI: 1.06–3.69; P=0.03) were independent prognostic factors (Table III).
Table II.Univariate and multivariate analyses of clinicopathological factors and preoperative ALI for overall survival after propensity matched analysis. |
Table III.Univariate and multivariate analyses of clinicopathological factors and preoperative ALI for relapse-free survival after propensity matched analysis. |
Discussion
This research focused on ALI as a marker of inflammation and nutritional status in patients with GC undergoing curative resection. We examined its clinical utility by analyzing the association between preoperative ALI and survival. Our findings revealed a significantly poorer prognosis in terms of OS and RFS in the low-ALI group compared with that in the high-ALI group. Multivariate analysis further confirmed that low ALI was an independent poor prognostic indicator for both OS and RFS.
Initially, ALI was reported as a prognostic factor in patients with lung cancer, although recent studies have highlighted the prognostic value of ALI in various gastrointestinal malignancies postoperatively. In esophageal squamous cell carcinoma, retrospective studies demonstrated that lower ALI correlated with worse cancer-specific survival (CSS) and OS (24,25). Similarly, in colorectal cancer, patients with lower ALI exhibited poorer PFS and OS, with ALI identified as an independent prognostic factor in multivariate analyses (26,27). For GC, our results align with those of previous research, underscoring the potential of ALI as a significant prognostic marker postoperatively.
Several investigations have examined the prognostic significance of ALI in patients with GC after surgery, and these results are generally consistent with those of the present study. A retrospective study of 620 patients with GC after surgery showed that patients with low ALI had significantly poorer OS and DFS than that of those with high ALI (P<0.001 and P<0.001, respectively) (19). In multivariate analysis, ALI was an independent prognostic indicator for OS (P=0.006). A retrospective study of 615 patients with GC after surgery showed that patients with high ALI had significantly longer OS and DFS than that of those with low ALI (P<0.001 and P<0.001, respectively) (20). In multivariate analysis, ALI was an independent prognostic factor for OS and DFS (P=0.001 and P=0.009, respectively). A retrospective study of 1657 patients with GC after surgery showed that patients with low ALI had significantly worse OS and CSS than that of those with low ALI (P<0.001 and P=0.001, respectively) (21). In multivariate analysis, ALI was an independent prognostic factor for OS and DFS (P=0.01 and P=0.04, respectively). In light of these findings, this study is one of the few novel studies to identify ALI as a useful prognostic factor for patients with GC using propensity score matching analysis. Furthermore, our results support the robustness of ALI as a prognostic predictor and may lead to further large-scale prospective studies in the future.
The effectiveness of ALI as a prognostic tool likely stems from its incorporation of both BMI and NLR/Alb, reflecting a patient's inflammatory immune function and nutritional status. In GC, low BMI has been established as a prognostic factor for DFS, and a positive correlation exists between preoperative BMI and prognostic nutritional indices (28), thereby emphasizing the importance of nutritional and immune status in patient outcomes. Recently, NLR/Alb has also been reported to be an important prognostic factor in patients with GC (29,30). Therefore, interventions such as aggressive nutritional therapy and rehabilitation (31) may be possible in patients with low ALI, which may contribute to improved prognosis.
However, this study had some limitations. First, is its single-center and retrospective design. Second, the paucity of literature on ALI in GC left the optimal cutoff value undetermined. Further multicenter, prospective studies are required to validate the utility of ALI and establish a clinically relevant cutoff value. Third, the data set used was that of Japanese people only, thus no comparisons could be made between races.
In conclusion, preoperative ALI emerges as a potentially valuable prognostic tool in patients with GC undergoing radical surgery. Our study supports the notion that lower ALI levels are indicative of poorer survival outcomes, highlighting the need for further research to effectively integrate this marker into clinical practice.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
IH and TOs conceived and designed the study. IH, MT, SO, JM, SN, YM, KK, TA, TY, TOg, NY, AS, YR and TOs analyzed and interpreted the data. IH and TOs confirm the authenticity of all the raw data. IH and TOs prepared the draft manuscript and figures. IH, MT, SO, JO, SN, YM, KK, TA, TY, TOg, NY, AS, YR and TOs collected the data and performed the literature search. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the Kanagawa Cancer Center (approval no. 25Research-20), and all procedures were conducted following the Declaration of Helsinki in 1996. Written informed consent was obtained from all patients in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
ALI |
advanced lung cancer inflammation index |
|
BMI |
body mass index |
|
CI |
confidence interval |
|
HR |
hazard ratio |
|
pStage |
pathological stage |
|
TG |
total gastrectomy |
|
OS |
overall survival |
|
RFS |
relapse-free survival |
|
GC |
gastric cancer |
|
NLR/Alb |
neutrophil-to-lymphocyte ratio/albumin |
|
CSS |
cancer-specific survival |
References
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ishikawa D, Nishi M, Takasu C, Kashihara H, Tokunaga T, Higashijima J, Yoshikawa K and Shimada MT: The role of neutrophil-to-lymphocyte ratio on the effect of CRT for patients with rectal cancer. In Vivo. 34:863–868. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Fukuda N, Toda K, Fujiwara YU, Wang X, Ohmoto A, Urasaki T, Hayashi N, Sato Y, Nakano K, Yunokawa M, et al: Neutrophil-to-lymphocyte ratio as a prognostic marker for anaplastic thyroid cancer treated with lenvatinib. In Vivo. 34:2859–2864. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Hayama T, Ozawa T, Tsukamoto M, Fukushima Y, Shimada R, Nozawa K, Matsuda K, Fujii S, Fukagawa T and Hashiguchi Y: Predicting overall survival using preoperative nutritional and inflammation status for colorectal cancer. In Vivo. 36:450–457. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Tsukioka T, Izumi N, Komatsu H, Inoue H, Ito R, Suzuki S and Nishiyama N: Elevation of neutrophil-to-lymphocyte ratio is a significant poor prognostic factor in completely resected centrally located lung squamous cell carcinoma. In Vivo. 36:2303–2307. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Hsueh WH, Hsueh SW, Yeh KY, Hung YS, Ho MM, Lin SY, Tseng CK, Hung CY and Chou WC: Albumin and neutrophil-to-lymphocyte ratio score in neoadjuvant concurrent chemoradiotherapy for esophageal cancer: Comparison with prognostic nutritional index. In Vivo. 36:2400–2408. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Hsu CC, Chou WC, Hung YS, Lin SY, Hung CY, Yeh KY, Wang HM and Lu CH: Predictive value of albumin and neutrophil-to-lymphocyte ratio score for treatment completeness and safety profiles in patients with head and neck cancer receiving definitive concurrent chemoradiotherapy. In Vivo. 36:2875–2883. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Aoyama T, Kazama K, Maezawa Y and Hara K: Usefulness of nutrition and inflammation assessment tools in esophageal cancer treatment. In Vivo. 37:22–35. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Deng Q, He B, Liu X, Yue J, Ying H, Pan Y, Sun H, Chen J, Wang F, Gao T, et al: Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med. 13:662015. View Article : Google Scholar : PubMed/NCBI | |
|
Aoyama T, Hara K, Kazama K and Maezawa Y: Clinical impact of nutrition and inflammation assessment tools in gastric cancer treatment. Anticancer Res. 42:5167–5180. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Hirahara N, Matsubara T, Kaji S, Hayashi H, Sasaki Y, Kawakami K, Hyakudomi R, Yamamoto T and Tajima Y: Novel inflammation-combined prognostic index to predict survival outcomes in patients with gastric cancer. Oncotarget. 14:71–82. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Jafri SH, Shi R and Mills G: Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): A retrospective review. BMC Cancer. 13:1582013. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Q, Chen S and Feng JF: A novel inflammation-based prognostic index for patients with esophageal squamous cell carcinoma: Neutrophil lymphocyte ratio/albumin ratio. Oncotarget. 8:103535–103542. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Jun DH, Kim BJ, Park JH, Kim JG, Chi KC, Park JM, Kim MK and Kang H: Preoperative body mass index maydetermine the prognosis of advanced gastric cancer. Nutr Cancer. 68:1295–1300. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
He X, Zhou T, Yang Y, Hong S, Zhan J, Hu Z, Fang W, Qin T, Ma Y, Zhao Y, et al: Advanced lung cancer inflammation index, a new prognostic score, predicts outcome in patients with small-cell lung cancer. Clin Lung Cancer. 16:e165–e171. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Park YH, Yi HG, Lee MH, Kim CS and Lim JH: Prognostic value of the pretreatment advanced lung cancer inflammation index (ALI) in diffuse large B cell lymphoma patients treated with R-CHOP chemotherapy. Acta Haematol. 137:76–85. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Jank BJ, Kadletz L, Schnöll J, Selzer E, Perisanidis C and Heiduschka G: Prognostic value of advanced lung cancer inflammation index in head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 276:1487–1492. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Yin C, Toiyama Y, Okugawa Y, Omura Y, Kusunoki Y, Kusunoki K, Imaoka Y, Yasuda H, Ohi M and Kusunoki M: Clinical significance of advanced lung cancer inflammation index, a nutritional and inflammation index, in gastric cancer patients after surgical resection: A propensity score matching analysis. Clin Nutr. 40:1130–1136. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X, Wang D, Sun T, Li W and Dang C: Advanced lung cancer inflammation index (ALI) predicts prognosis of patients with gastric cancer after surgical resection. BMC Cancer. 22:6842022. View Article : Google Scholar : PubMed/NCBI | |
|
Chen H, Zhang F, Luo D, Guo J, Lin Y, Chen S, Yin S, Chen X, Peng J and Lian L: Advanced lung cancer inflammation index predicts the outcomes of patients with non-metastatic gastric cancer after radical surgical resection. J Gastrointest Oncol. 14:85–96. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Huo C, Liu Y, Xie F, Zhao L, Huang H and Feng Q: Advanced lung cancer inflammation index predicts the outcomes of patients with non-metastatic gastric cancer after radical surgical resection. J Gastrointest Oncol. 14:1653–1654. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang X, Liu X, Dai H and Jia J: Peripheral blood nutrient indices as biomarkers for anti-PD-1 therapy efficacy and prognosis in patients with advanced gastric cancer. Oncol Lett. 26:3972023. View Article : Google Scholar : PubMed/NCBI | |
|
Feng JF, Huang Y and Chen QX: A new inflammation index is useful for patients with esophageal squamous cell carcinoma. Onco Targets Ther. 7:1811–1815. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Tan X, Peng H, Gu P, Chen M and Wang Y: Prognostic significance of the L3 skeletal muscle index and advanced lung cancer inflammation index in elderly patients with esophageal cancer. Cancer Manag Res. 13:3133–3143. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Kusunoki K, Toiyama Y, Okugawa Y, Yamamoto A, Omura Y, Ohi M, Araki T and Kusunoki M: Advanced lung cancer inflammation index predicts outcomes of patients with colorectal cancer after surgical resection. Dis Colon Rectum. 63:1242–1250. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang B, Li ZW, Tong Y, Yuan C, Liu XY, Wei ZQ, Zhang W and Peng D: The predictive value of advanced lung cancer inflammation index for short-term outcomes and prognosis of colorectal cancer patients who underwent radical surgery. Int J Clin Oncol. 28:1616–1624. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Park SH, Lee S, Song JH, Choi S, Cho M, Kwon IG, Son T, Kim HI, Cheong JH, Hyung WJ, et al: Prognostic significance of body mass index and prognostic nutritional index in stage II/III gastric cancer. Eur J Surg Oncol. 46:620–625. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Hashimoto I, Kano K, Onuma S, Suematsu H, Nagasawa S, Kanematsu K, Furusawa K, Hamaguchi T, Watanabe M, Hayashi K, et al: Clinical significance of neutrophil-to-lymphocyte ratio/serum albumin ratio in patients with metastatic gastric or gastroesophageal junction cancer administered trifluridine/tipiracil. Anticancer Res. 43:1689–1697. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Onuma S, Hashimoto I, Suematsu H, Nagasawa S, Kanematsu K, Aoyama T, Yamada T, Rino Y, Ogata T and Oshima T: Clinical effects of the neutrophil-to-lymphocyte ratio/serum albumin ratio in patients with gastric cancer after gastrectomy. J Pers Med. 13:4322023. View Article : Google Scholar : PubMed/NCBI | |
|
Yamamoto K, Nagatsuma Y, Fukuda Y, Hirao M, Nishikawa K, Miyamoto A, Ikeda M, Nakamori S, Sekimoto M, Fujitani K and Tsujinaka T: Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer. 20:913–918. 2017. View Article : Google Scholar : PubMed/NCBI |